E-Beam vs. EO Sterilization
Looking to elevate your sterilization process?
E-Beam delivers unmatched speed and sustainability.
E-Beam and Ethylene Oxide Side By Side Comparison
| Capabilities | ||
|---|---|---|
| Sterilization Source | Grid electricity | Ethylene Oxide gas |
| Technology Maturity | High | High |
| Process Quantity | Boxes | Pallet |
| Processing Time | Seconds | Days |
| Sustainability & Environmental Impact | As clean as the electricity used to power the system | Toxic gas must be contained EPA legislating new limits now |
| Benefits | • Extremely efficient: sustainably process most volume / year • Gentlest on materials as compared to other forms of radiation sterilization • Best $ / capacity available | • Chemical sterilant with excellent absorption • Wide materials compatibility |
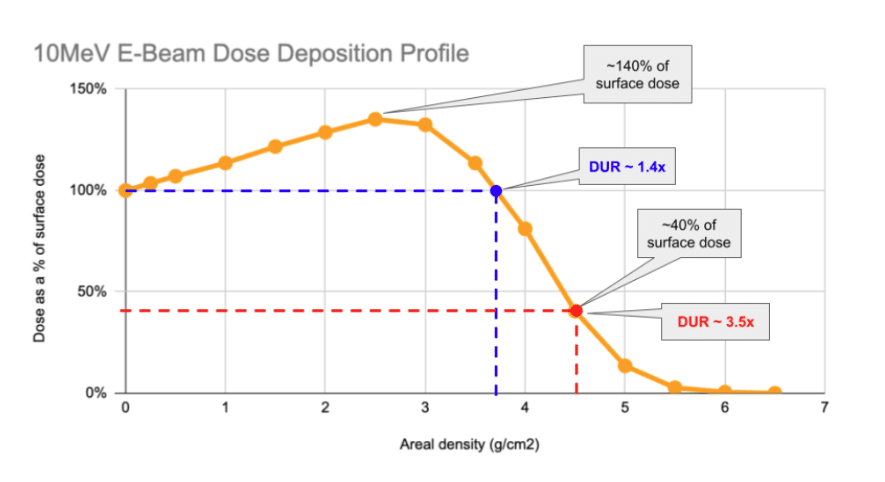
| Limitations | • Product requiring tight DURs can be challenging • Large / dense products can be challenging | • Residuals problematic • Litigation risk • Environmental risk • New regulatory risk |
| Outlook | Growth: efficient, sustainable technology | Slow phase-out over decades due to Environmental & litigation risk |
Advantages
E-Beam
E-Beam offers rapid sterilization, with most products receiving their dose in just minutes or even seconds. This speed translates to shorter turnaround times, making E-Beam ideal for fast-paced, high-volume operations.
E-Beam systems can process large volumes of products each day. When medical devices are packaged for optimal E-Beam compatibility, they can achieve superior throughput and often a lower overall cost than EO, which involves more handling and longer processing times.
E-Beam sterilization facilities are stable and long-lived, powered by clean grid electricity. They do not use or produce harmful chemicals like EO, making them a more environmentally friendly option. There’s no need for special handling or aeration processes, which reduces potential supply chain disruptions and improves reliability.
EtO
Probably the best part of EO for most users is that it is gentle on soft polymers such as PTFE (Teflon) and other specialty materials that frequently can be part of modern medical devices.
EO, especially when combined with modest amounts of heat and humidity is an incredibly penetrative gas that can be used to access all of the surface regions in most products.
Most high-volume EO facilities offer multiple chambers capable of processing numerous pallets at once. While the dwell and aeration times for EO are much longer than the processing time required for E-Beam, the highly parallel nature of a large EO facility does offer high levels of throughput.


What NextBeam Customers Say
“NextBeam has changed the game for us with their speed, accuracy, and reliable operation. Product comes back on time and quality has been superb.”
Frequently Asked Questions About E-Beam & EO Sterilization
EO is a toxic gas and requires careful handling to ensure the safety of workers and the environment. Sterilized products must undergo aeration to remove any residual EO. E-Beam, on the other hand, is a clean process that doesn’t involve harmful chemicals or leave residues behind.
While EO is effective, it carries health risks due to its toxicity and the need for strict ventilation measures. E-Beam is a safer alternative, using only electricity and producing no toxic byproducts.
E-Beam sterilization is much faster, often taking just minutes. EO, by comparison, takes several hours because of the need for exposure and aeration time.
EO works well for materials sensitive to radiation, like certain fabrics and Teflon. E-Beam is ideal for most medical devices and materials, offering rapid processing without the need for chemical sterilization.
EO requires careful disposal of toxic emissions and has a higher environmental impact. E-Beam is more eco-friendly, as it uses clean electricity and produces no hazardous waste.
A Comprehensive Breakdown Between
E-Beam and Ethylene Oxide
- Both E-Beam and EO are compatible with many medical devices and products from other industries.
- E-Beam excels with low-density products (<0.2g/cm³) and is often a better choice for long-term durability and supply chain reliability.
- EO is recommended when a product contains materials that cannot withstand radiation, though this comes with drawbacks in terms of flexibility and industry practice.
- E-Beam and EO both deliver the same level of sterilization (SAL) when properly validated, following ISO standards.
- Both methods are effective, but E-Beam provides faster processing, making it a more efficient option for many products.
- EO has traditionally been cost-effective, but prices are rising due to facility closures and new environmental regulations.
- E-Beam is often more economical, especially for products designed to be compatible with it, and it may soon outpace EO as the lower-cost option.
- The FDA recognizes both EO and E-Beam as mature, Category A modalities. Transitions between EO and E-Beam are easier than many assume, often not requiring a new 510(k) if biocompatibility and performance remain unchanged.
- Manufacturers should consult the FDA’s guidelines for switching modalities.