Industries We Serve
Want to work with us?
Contact Us
→ Medical Device
→ Laboratory Supply
→ Food & Consumer
→ Packaging
→ Pharmaceutical & Bioprocessing
Take our quiz to understand if you’re a good fit for Electron Beam sterilization →
Medical Device Sterilization
Industries We Serve
Want to work with us?
Connect with a sterilization expert →
→ Food & Consumer
→ Packaging
→ Laboratory Supply
→ Pharmaceutical & Bioprocessing
Take our quiz to understand if you’re a good fit for Electron Beam sterilization →
Why Choose E-Beam?
More than we can fit on a page, so here are a few highlights for the medical device industry.
Precise and Efficient
Tap to Reveal How
Medical devices receive just a few seconds of precision irradiation dosing. E-Beam is simple to qualify: no EO or other chemical residuals are left on items or packaging, making them immediately safe to handle and ready for distribution. Our high-capacity, $20M facility allows us to process many truckloads daily, ensuring your product is on its way to consumers without delay.
Sustainable &
Safe
Tap to Reveal How
Conventional grid power serves as the basis of E-Beam sterilization and offers a greener option for sterilization and bioreduction. Compared to Gamma or Ethylene Oxide processes, E-Beam doesn’t require source materials that are dangerously carcinogenic or radioactive.
Enhanced Material Compatibility
Tap to Reveal How
For radiation sterilization-compatible medical devices, E-Beam offers the best possible materials compatibility, thanks to the rapid dose delivery E-Beam makes possible: Minimal time under beam spares products from being degraded over hours spent in the highly oxidative environment that is a gamma bunker.
Reliable
Results
Tap to Reveal How
Qualified products are safely irradiated in large quantities with reliable precision in our fully ISO-certified process. E-Beam sterilizes without heat or prolonged and oxidative radiation exposure, effectively maintaining the quality and integrity of medical devices inside of their sterile barrier packaging.
Medical Device Applications
Leverage E-Beam’s best possible material compatibility and FDA-recognized maturity to safeguard your products.
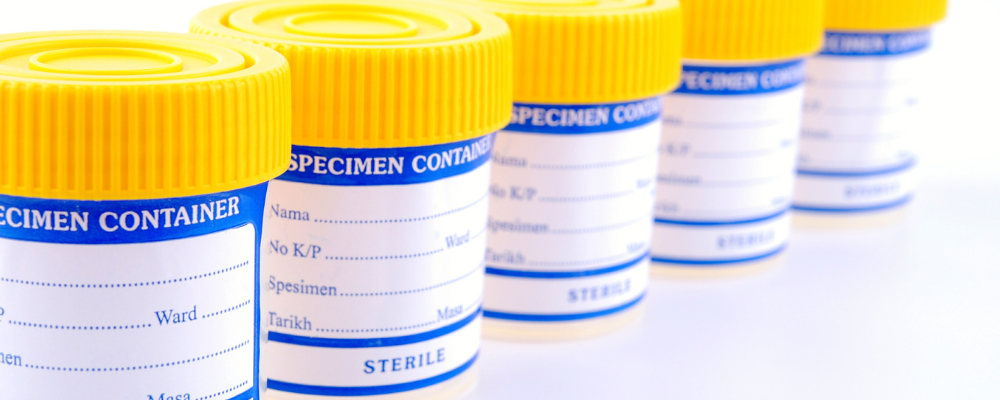
High-Volume Single-Use Devices
- Rapidly sterilize truckloads of high-volume single use devices: from sterile cotton swabs to specimen collection cups, E-Beam is a highly reliable and efficient method for large volume sterilization
- Eliminate longterm concerns around risks present with legacy modalities: litigation risk for EO and escalating costs for both EO and Gamma processing.
Highly-Engineered Devices
- For lower-volume, high-value devices such as implantable medical devices, E-Beam’s precision, reliability, and rapid dose delivery assure that the customer can achieve the best possible sterilization outcome.
- Eliminate concerns around complex qualification processes (e.g. EO residuals) and future-proof expensive products by designing for E-Beam upfront.
The NextBeam Standard
NextBeam offers an unparalleled electron beam sterilization service that raises the industry standard.

Unparalleled Sterilization Assurance
Experience industry-leading sterilization with NextBeam, boasting a remarkable <0.5% inventory scrap rate for the utmost product safety.
Efficient Qualification Process
Skip the wait! Our dedicated team ensures your product compatibility with E-Beam technology in less than a week, providing detailed dose maps and quality reports.


Strategic Location for Swift Delivery
Strategically positioned for convenience, NextBeam’s midwestern facility location ensures your products spend less time in transit, hitting the shelves faster for increased market presence.
Leverage medical device-grade quality for your business
Our quality system is fully compliant with ISO 13485, ISO 11137, and ISO 9001 for medical device manufacturing, radiation sterilization, and quality management systems.






Let's connect
Complete this form, or email hello@nextbeam.com, and a sterilization expert will get back to you within one business day.
FAQs
- Precise and reliable dose delivery assures the integrity and safety of medical devices.
- Efficient E-Beam technology helps deliver highest quality yet cost-effective outcomes.
- NextBeam meets or exceeds rigorous ISO 13485, ISO 11137, and ISO 9001 measures.
- Individual products usually only need a few seconds of precisely calculated irradiation, making processing bulk products and large shipments highly efficient. Once irradiated, products are immediately safe for handling: E-Beam leaves no trace of chemicals or radiation.
- NextBeam’s state-of-the-art facility is designed for high throughput and typically processes product in under 5 days. Expedite options can be made available.