How We Work
E-Beam Sterilization: A simple 2-step process

Step 1 — Dose Mapping & Qualification
We assess your product and requirements. If appropriate, we test-irradiate your product and evaluate results to develop a custom process for each SKU.

Step 2 — Routine Processing
Once qualified, we’ll provide you with an estimate for processing services according to your volume and commercial requirements.

What is Dose Mapping and Qualification?
For E-Beam processing, Dose Mapping and Qualification is the crucial process of determining the optimal electron beam dosage and process parameters for a specific product, ensuring that the radiation dose required for successful sterilization can be delivered within specification and at maximum efficiency. At NextBeam, we also include a processing optimization study whenever possible.

Typically completed within 5 days from receipt of the product
All NextBeam qualifications include both dose mapping (per ISO 11137 standard) as well as process optimization when possible

We only recommend customers undertake qualification when 90%+ confident in successful completion

Comprehensive reports are provided to confirm your product’s preparedness for volume processing
Analysis
We analyze sterility requirements and primary data to assess product suitability for E-Beam. If suitable, we request a sample.

Preparation
Each sample undergoes meticulous preparation. We place dosimeters to measure dose distribution within the product.

Dosing & Data Analysis
We irradiate at least three samples. Our lab extracts data from dosimeters and creates a statistical summary of dose distribution.

Reporting
We generate a comprehensive report summarizing the experiment design and results, confirming process parameters.

What is Routine Processing?
Routine Processing refers to the regular or large-scale application of electron beam technology for sterilization or other purposes. Once a product completes the Dose Mapping & Qualification process, it is ready for routine processing using E-Beam radiation.

Processing typically completed in 5 days or less from receipt

E-Beam is clean, sustainable technology: an all-electric process with that produces no harmful / radioactive materials
E-Beam tech delivers doses in seconds and is gentler on product than gamma

Scrap rate below 0.1% and dropping: Minimal waste; maximum efficiency

Receipt
Once a product qualifies, we’re ready to process it. We receive customer products in quantities ranging from a single pallet to multiple truckloads.

Processing
We irradiate the product using the tailored parameters determined during qualification, ensuring optimal results.

Quality Control (QC)
Our on-site dosimetry lab confirms that the received product is dosed within specification, maintaining high-quality standards.

Shipment
We efficiently prepare and load the processed product for pickup by the customer’s carrier. Comprehensive quality records are provided electronically.
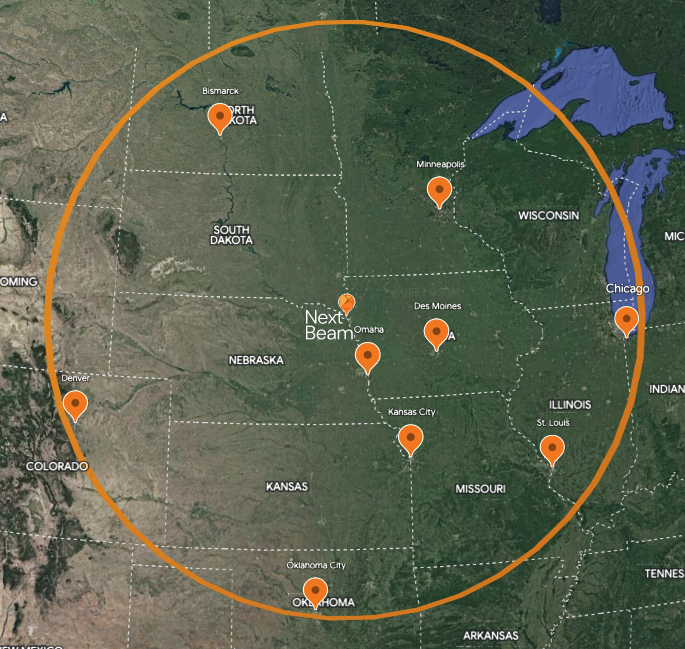
NextBeam is strategically positioned for your convenience, situated within a day’s drive of major cities such as Chicago, Minneapolis, Denver, St. Louis, Kansas City, and more, catering to both large and small businesses.
Subscribe to our newsletter to learn about future expansions.
Let's Connect
Complete this form, or email hello@nextbeam.com, and a sterilization expert will get back to you within one business day.
FAQs
How much volume can you process?
Our process is designed to support a wide range of volumes, from a single pallet to multiple truckloads of product.
What is the maximum product size you can process?
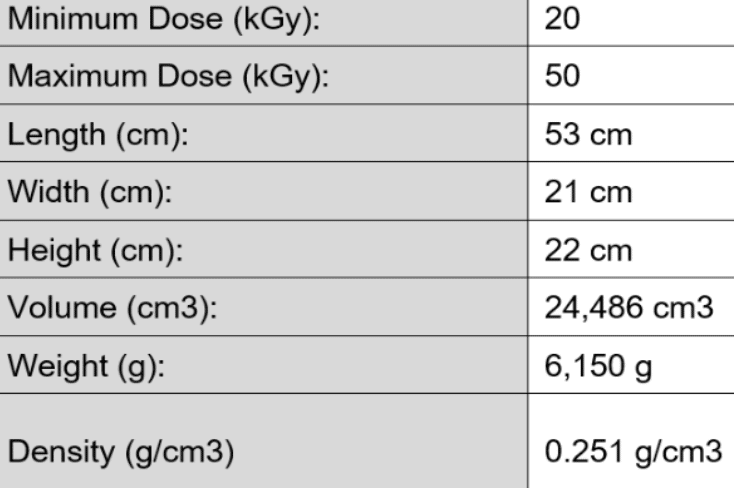
The maximum standard product envelope is 50” x 30” x 48”. If you have larger needs, please contact us for further assistance.
What industries do you serve?
We serve a diverse range of industries, including medical devices, lab animal science, laboratory supply, and more.
We Meet or Exceed Global Quality Sterilization Standards


Quality system accredited to ISO 9001:2015, Quality management systems

Sterilization working group member (WG02)

E61 Radiation processing committee member

FDA-registered establishment