“They always say time changes things, but you actually have to change them yourself.”
— Andy Warhol
For better or worse, we live in a world where the ethylene oxide (EO) sterilization industry is facing significant threats from litigation and increased regulatory scrutiny over its safety and sustainability in the communities where these facilities operate. We’ve written about the future of EO before but in this article, we explore more of the differences between E-Beam and EO for customers looking to sterilize / bioreduce their products.
A Little History
E-Beam (first commercial use in 1950s) is a technology that is nearly as old as EO (initial sterilization patent in 1938). In this era, steam sterilization dominated the fledgling medical device industry – while appropriate for steel instruments, the increase in plastic and other medical devices that could not tolerate high temperatures drove the growth of EO. With little knowledge of the environmental risks of EO, it proliferated strongly over the next 50 years.
E-Beam’s early years were marked by low-energy beams with reliability limits. Fortunately, especially over the past 20 years, the reliability, and scalability of E-Beam technology has markedly improved: modern systems are designed to operate with over 95% uptime and have modern digital control systems that enable precise logging, process interrupt recovery capability, and a host of other options that help assure consistent, high-quality operation.
Ethylene Oxide vs. Electron Beam Sterilization
| EtO | E-Beam | |
|---|---|---|
| Source | ||
| Technology Maturity | High | High |
| Process quantity | Pallet | Boxes |
| Processing time | Days | Seconds |
| Sustainability & Environmental impact | Toxic gas must be contained EPA legislating new limits now |
As clean as the electricity used to power the system |
| Benefits | • Chemical sterilant with excellent absorption • Wide materials compatibility |
• Extremely efficient: sustainably process most volume / year • Best $ / capacity available |
| Limitations | • Residuals problematic • Litigation risk • Environmental risk • New regulatory risk |
• Products requiring tight DURs are challenging • Large / dense products can be challenging |
| Outlook | ❌ Slow phase-out over decades due to Environmental & litigation risk |
✅ Growth: Efficient, sustainable technology |
Electron Beam (E-Beam) Sterilization Advantages
At NextBeam, customers frequently reach out to us with similar stories: their legacy EO or Gamma supply has either gone away or significantly increased in expense. Some are concerned with either the litigation or environmental risk around EO.
Whatever their motivation, E-Beam offers major advantages to users:
- Scalability: Modern E-Beam facilities deliver impressive scale: they can process many truckloads of product each day.
- Speed: E-Beam is fast: most “shipper boxes” of materials receive their sterilization dose in just a few minutes, if not seconds. Accordingly – turn time can be short.
- Sustainability: E-Beam systems run off of commercial grid power and have are either zero or very low emission (ozone is the only byproduct).
- Safety: E-Beam does not leave any potentially harmful chemical residuals on processed products. Additionally, E-Beam is safe: there is no explosion risk.
- Economics: For many products – especially medical devices – E-Beam is the most economical modality to process.

Ethylene Oxide Sterilization Advantages
At NextBeam we’re quick to point out that EO, while beset by environmental and legal issues that are unlikely to ever completely disappear, is an incredibly high-performance sterilization technology offering a number of advantages for users:
- Material Compatibility: Probably the best part of EO for most users is that it is gentle on soft polymers such as PTFE (Teflon) and other specialty materials that frequently can be part of modern medical devices.
- Penetration: EO, especially when combined with modest amounts of heat and humidity is an incredibly penetrative gas that can be used to access all of the surface regions in most products.
- Scalability: Most high-volume EO facilities offer multiple chambers capable of processing multiple pallets at once. While the dwell and aeration times for EO are much longer than the processing time required for E-Beam, the highly parallel nature of a large EO facility does offer high levels of throughput.
Material Compatibility
Which industries/kinds of products/materials does E-Beam do well in, and what about EO?
Both E-Beam and EO are widely (but not always) compatible with most medical devices and a host of products from other industries seeking either sterilization or bioburden reduction (“bioreduction.”)
AAMI’S TIR 17 offers a great overview of how materials degrade in varying amounts of radiation dose. We have also published a much shorter and simplified overview of compatible materials.
In any case – E-Beam tends to be exceptionally efficient for products that are of low-ish density (< 0.2g/cm3). If you don’t understand how dose is determined during a sterilization validation, this article can help.
We of course are biased, but only recommend EO only to customers whose products incorporate materials that are fundamentally incompatible with radiation, with no alternatives available today, or where there may be available alternatives but the timeline to make these changes is very long. Wherever possible we encourage customers to make “healthy choices” for the durability of their supply chains, leverage in future procurement conversations, and avoidance of litigation risk.
So Why Doesn’t Everyone Shift to E-Beam Immediately?
We view EO’s grip on the industry as being the result of long practice: for many decades EO was high-performance, relatively inexpensive, and was flexible enough to accommodate a a wide variety of devices. Indeed, this flexibility was so good that it enabled product developers to not consider sterilization until very late in the development process.
E-Beam can provide fast and economic sterilization for many products, and can be set up most efficiently when contemplated
earlier in the lifecycle of the product. The device material selection, geometry, package design, and overpack design are all important features to consider for compatibility with E-Beam.
The good news is that there are many simple guidelines (and emerging tools) that can help device and packaging engineers plan upfront for sterilization, and this planning is often very simple in practice.
How Effective Are These? Is There a Difference Between EO and E-Beam?
Both EO and E-Beam, should be equally effective in delivering a target sterility assurance level (SAL), assuming a product is properly qualified per the standards described in ISO 11135 and ISO 11137 (standards for EO and Radiation sterilization of healthcare products, respectively). Among other things, these standards describe how a sterilization validation should be structured for each modality to achieve a target SAL.
What About Pricing?
Historically EO has been inexpensive. However, pricing is increasing: 10 of the 96 EO facilities tracked by the EPA closed between 2022 and 2023. With the EPA’s final ruling requiring additional environmental controls and plant safety upgrades, we expect that the cost of EO will only climb: the direct cost of these upgrades will need to be absorbed into pricing and, perhaps more perniciously, we expect that there will be further facility closures: only large and well-capitalized competitors will be able to survive.
We expect that E-Beam pricing either is or will be lowest cost over time, especially for products designed to be compatible with E-Beam.
Are There Differences in Regulatory Compliance? What if I Want to Switch from EO to E-Beam?
The FDA considers both EO and E-Beam to be “Established Category A” modalities, meaning that they are the most mature modalities that the FDA considers. Further, the FDA is more permissive than most people think when it comes to allowing transitions between EO and E-Beam and has codified its thinking on changing modalities in a flowchart in the document “Deciding When to Submit a 510(k) for a Change to an Existing Device.” The below chart is taken from page 24 with our (colored) annotations:
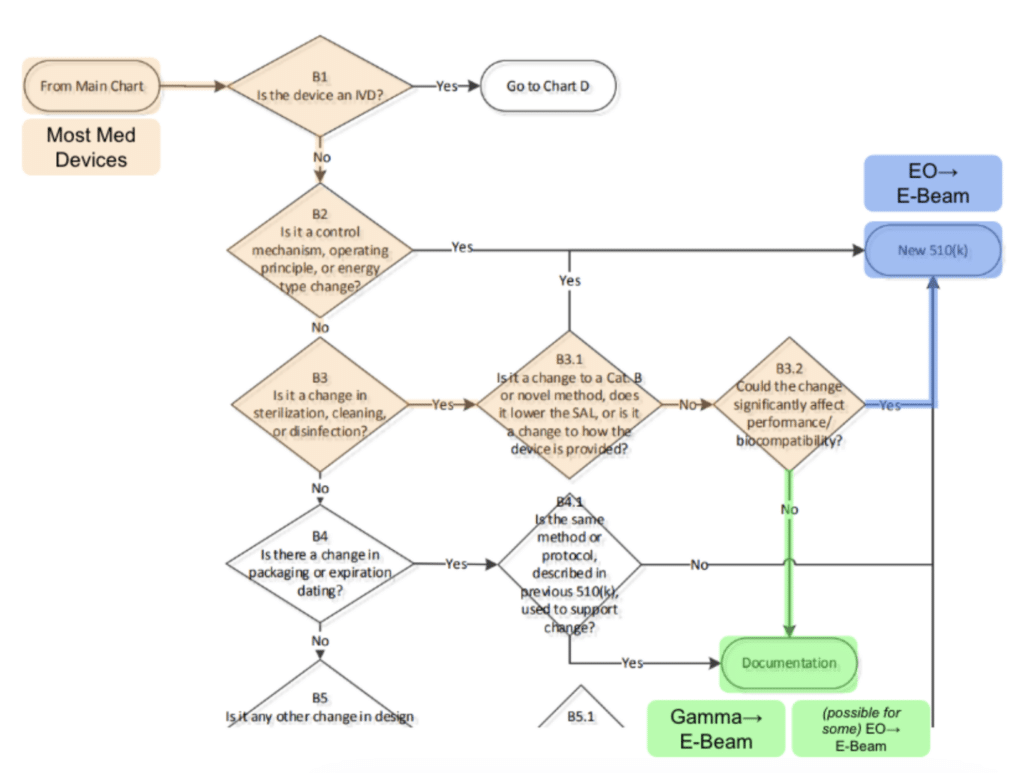
We interpret B3.2 in this chart to indicate that manufacturers can, for EO → E-Beam transfers, perform their own analysis and/or testing to determine whether or not the new modality would negatively impact biocompatibility or cause any change in the performance of the device or sterile barrier system. If the answer is no, then the change is doable without the need for a new 510(k) submission.
I Want to See if I Can Shift my Device From EO to E-Beam. How Do I Get Started?
The specific product’s engineering team is best positioned to understand the materials in the product, it’s specific performance requirements, and how to test/validate these.
From a E-Beam sterilization validation standpoint, we recommend dose mapping and performing a max dose study as early as possible: these are fairly inexpensive and fast ways to eliminate risk.
At NextBeam, we typically recommend a consultation with our technical team and the device’s design team as a first, simple step before any testing is performed. There is no charge for this consultation. Contact us today to get started.