The times they are a changin’
– Bob Dylan
Today we are in the beginning of an epochal shift in how sterile / bioreduced products are treated. Traditional methods like EO and Gamma are, for various reasons, not likely to continue to scale in the US. Beam-based technologies are the future: precise and sustainable.
But, unfortunately, E-Beam is not a silver bullet for every product. We put together this short guide to help users understand where E-Beam excels – and where it doesn’t.
3 Key Factors to Consider
While E-Beam technology is in practice complex to accurately model, back-of-the-napkin math can offer an “80%” estimate for how likely products are to be E-Beam compatible. Three key factors should be considered:
(1) Material Compatibility
Material compatibility is typically a “knockout” question to assess. The below chart lists materials commonly used in medical device construction that are compromised by radiation above certain dose levels.
Typically, if a device has previously been qualified in Gamma, E-Beam sterilization tends to be equivalent-to-gentler on the device due to E-Beam’s rapid delivery vs Gamma (seconds vs hours). This is due to minimizing the amount of time that the product is kept in an ozone-rich, highly-oxidative environment.
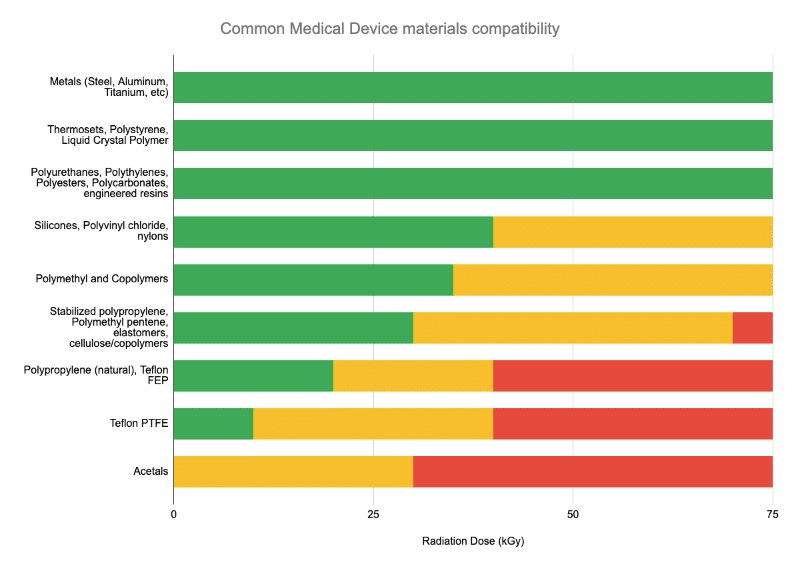
Many – but certainly not all – medical devices are qualified for radiation sterilization in the sub-30kGy range in the chart shown above.
So if the materials in your product are in the “green” range above – your product is a candidate for all types of radiation sterilization – E-Beam, Gamma, and X-ray.
(2) Product Areal Density
High-energy 10MeV electrons, on average, penetrate a certain, predictable amount of mass. While it’s typical to calculate average product densities when considering shipping logistics, etc, these volumetric densities (expressed in g / cm3) are not necessarily the limiting factor for E-Beam. Rather – the amount of mass under the beam is, which is expressed in areal density, or g / cm2.
The diagram below helps show the difference between volumetric and areal density:
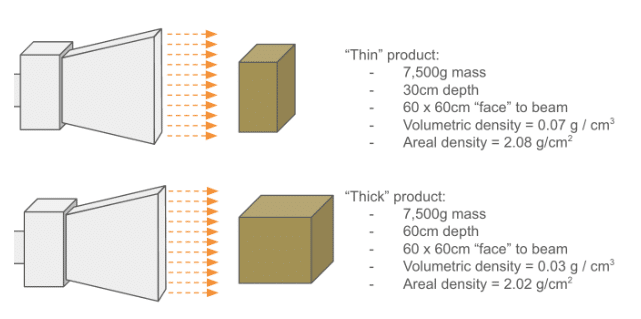
Also remember that if the product has different L x W x D dimensions, the areal density can be different for each “face” of the box. Shooting at the largest face of the box produces the lowest areal density.
Products with areal densities < 8 g/cm2 are generally compatible with E-Beam processing. But it’s important to remember that these are only average numbers: density varies inside of a box, which, in the case of medical devices, contains air, packaging material, and the device itself. Ultimately, performing a proper dose map assures that a product can be successfully irradiated within specification.
(3) Product Dose Uniformity Ratio
When qualifying a product for radiation sterilization per the processes outlined in ISO 11137, a minimum and maximum dose must be established. These are typically dependent on two tests:
Min dose (Dmin , sometimes called the sterilization dose, or Dster) – Dmin is determined based on 2 factors:
- What the target sterility assurance level (SAL) is for the product. Typically, for medical devices, a less-than 1-in-1-million probability of a living pathogen on a single device after sterilization.
- How microbiologically “clean” the product is at the end of its manufacturing process. This is typically assessed by a 3rd party laboratory, and is quantified in terms of colony-forming units (CFUs) per device.
Minimum dose is the minimum amount of radiation that must be applied to the device to ensure that the target SAL is met.
Maximum dose (Dmax) – Dmax is set through destructive testing: while there are different approaches to determining the true maximum dose, in essence, the idea is to determine a “safe” maximum value where the product being irradiated will not be damaged or have its functionality compromised.
The Dose Uniformity Ratio (DUR) is the ratio of the above: Dmax / Dmin and provides the “window” of permissible dose that the product requires.
One of the analogies that we like to use here is that low DURs are tough to achieve in the same way that achieving uniform temperature is when cooking a thick steak: getting dose evenly through the product (like getting heat evenly through the steak) isn’t possible – what is possible is achieving a target DUR within spec – just like the cooking the steak such that the rarest and most well-done pieces of it are still within the range of what a diner prefers.

Continuing the analogy: like steaks, products that are very thick/dense (and especially products that are highly variable in density) tend to be more difficult to dose within a tight DUR, so if a high (DUR > 3 or more) is possible to qualify in testing, more flexibility in packaging and choice of radiation modality is possible.
Putting It All Together
We developed the E-Beam Suitability Calculator to help professionals from the medical device and other industries rapidly conduct a first-pass evaluation of all of these factors… in less than 5 minutes. While the results are not absolute, they provide good guidance in the majority of cases.
Ultimately, these factors are pieces of a larger and more technically complex story that includes additional factors such as processing efficiency and other considerations. We’re always happy to hear from customers with complex situations and questions. Contact us for a no-obligation review of your product.