With at least 10 EO sterilization facility site closures in the last 5 years, and more expected in the future, customers are right to evaluate alternatives to legacy sterilization modalities like EO.
EO processing facility closures have not only lead to longer lead times for routine sterilization, but also an inability to quickly bring products to market or revalidate existing ones. The validation process for E-Beam is notably quicker and less costly, making it an attractive option.
Why Are We Seeing EO Validation Timelines Lengthen?
EO validations normally take longer than E-Beam validations for two reasons:
- Dose mapping in E-Beam is a rapid process that does not require any biological indicators
- E-Beam requires no residual testing
Running a full EO validation, which includes fractionals, 3-4 half cycles, and 3 full cycles (per ISO 11135), would take a minimum of 6 months under ideal conditions. The reality is that there are many variables that can cause delays.
Now, with the recent closures of EO facilities, the delays are becoming even more significant for several reasons:
- Lack of capacity, given facility closures, to execute non-production validation work
- Many users are now implementing EO cycle redesigns to comply with their sterilizers’ internal programs to reduce EO concentrations per cycle
We are now hearing from customers that EO validations in contract sterilizers – formerly 6-month processes – can now stretch beyond 18 months. Customers may find themselves waiting 3-6 months for a subject matter expert to take on their project, and then can potentially wait another 6 – 18 months depending on the amount of cycle work and redesign required.
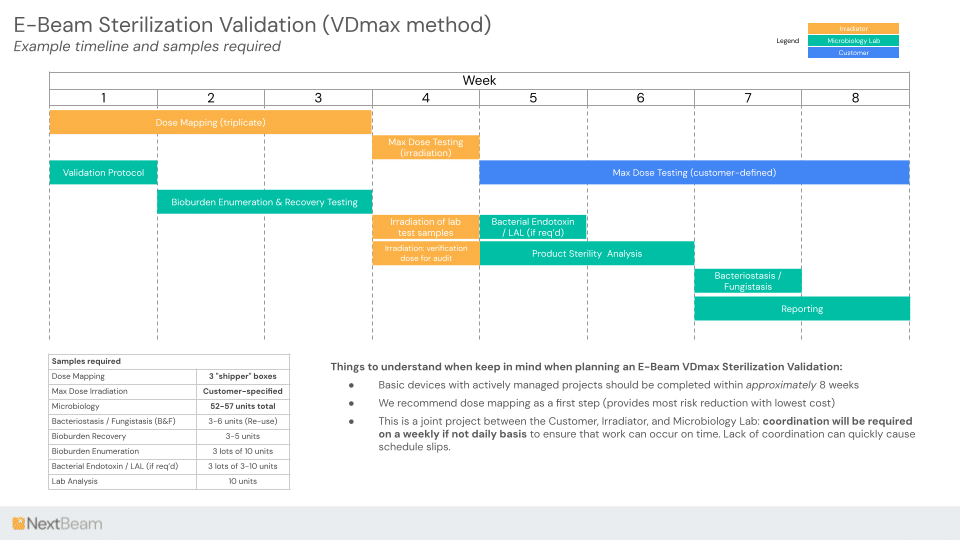
Speed-to-validation is one of the areas where E-Beam typically has an advantage over EO. ISO 11137 validations can be done in as little as 8 weeks.
What About the Cost of Validation?
Depending on the size of the chamber, EO Validations can be 5x (or more) the cost of an E-Beam validation, which highlights another difference between the two: typically, E-Beam’s high-speed but largely serial process (e.g. one of several boxes at a time) costs less to validate versus an EO validation, where a large chamber would need to be evaluated.
Between the added expense and longer lead times (especially in today’s environment) to validate an EO cycle, it is a no-brainer that many large companies are finding more ways to migrate products away from EO into faster, more sustainable technology like E-Beam.
Validation Criteria Comparison: EO Sterilization vs. E-Beam Sterilization
| EO Sterilization | E-Beam Sterilization | |
|---|---|---|
| Initial Engagement | 3 - 6 months | Immediate |
| Validation Process | 6 - 18 months | 6 - 8 weeks |
| Total Time | 12 months+ | 7 to 11 weeks |
| Cost | High | Less than EO ¼ of cost or less |
| Steps Involved |
1. Facility assessment and assignment of SME. Getting an SME can take months pending workload. 2. Cycle development or optimization and execution of cycle work (again, backlogs at sterilizers significantly drive this lead time out) 3. Biological and chemical indicator testing 4. Final Reporting and implementation of Validation |
1. Preliminary product and packaging assessment 2. Material compatibility testing 3. Dose mapping and distribution 4. Sterility Validation per ISO 11137. These steps can be overlapped with steps 2 & 3. 5. Implementation of Validated Final process |
EO Is Not Evil
At NextBeam, we view EO as a very high-performance sterilization technology that customers naturally gravitated towards given its historical low-cost and availability. Like E-Beam, the medical device industry has relied on it for decades. But the world has changed, and we view EO as a modality that should be selected because the product cannot be sterilized in a more sustainable way. Fortunately, E-Beam is well-positioned as a sterilization technology for many products that have traditionally been sterilized in EO.
I Want To See if I Can Shift My Device From EO to E-Beam. How Do I Get Started?
The device’s engineering team is best positioned to understand the materials in the device, its specific performance requirements, and how to test/validate these.
From a E-Beam sterilization validation standpoint, we advise clients to dose map and perform a max dose study as early as possible: these are fairly inexpensive and fast ways to eliminate risk.
At NextBeam, we typically recommend a consultation with our technical team and the device’s design team as a first, simple step before any testing is performed. There is no charge for this consultation. Contact us today to get started.
Additional Articles We Think You Might Like
Have a question? Speak with a sterilization expert today, at your own convenience.