Irradiating liquids with radiation for the purpose of sterilization or bioburden reduction tends to have similar limitations to the processing of solid products with several important caveats:
- Material compatibility is a concern (for products coming out of non-radiation modalities)
- Depth of penetration is a concern
- Single-sided processing is required (this is unique to liquids)
The third point is unique to liquids: to assure that the sterilization dose can be applied, we seek to irradiate the entire liquid in a single pass.
Typically, irradiation systems process double-sided, and, whether the mechanism to deliver double-sided dosing is two passes (with intermediate rotation) through a single-beamline bunker or a single pass (with no rotation) through a 2 beamline (opposing) system, the strong desire for delivering sterility is to assure that the sterilization dose required may be delivered through the entire depth of the product at once.
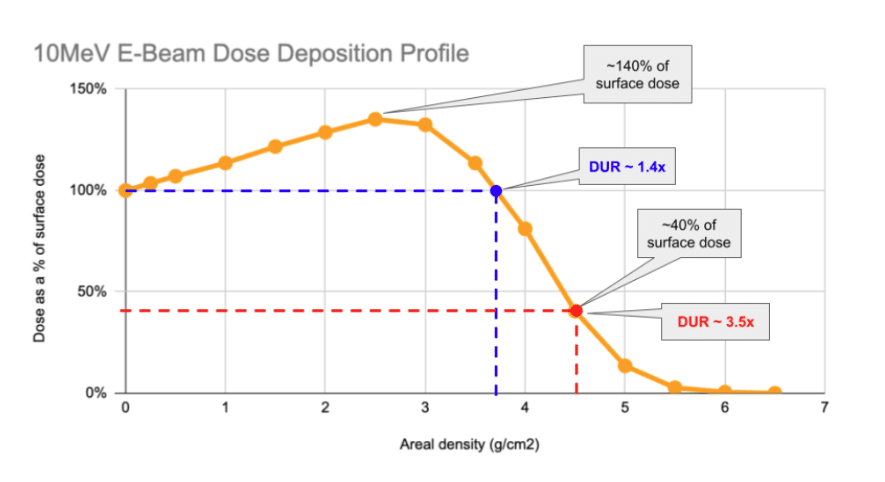
The above chart shows two examples of depth along the curve:
- The blue dot indicates the limit of a low DUR for a similar-to-water density liquid: as dose increases from 0 to around 2.5cm of depth, then decreases to equal the surface dose at depth = 0, the DUR moves from 1 up to ~1.4 and remains flat up to ~3.8cm of depth.
- Beyond this point, as illustrated by the red dot, even small increases in the depth of the water cause the DUR to ramp quickly. With the red dot example, a < 1cm increase in depth from 3.8 to 4.5cm has more than doubled the DUR.
Shown another way, the DUR for single-sided E-Beam begins to increase rapidly above 4 g/cm2 (or 4cm of water depth).
NextBeam Is Here to Help
These are the major concerns for liquid processing, though there can be some detail points on packaging / container types as well. In any case, our NextBeam experts are happy to provide a free consultation for your specific use case. Contact us today to get started.
Additional Articles We Think You Might Like
Have a question? Speak with a sterilization expert today, at your own convenience.