Electron Beam Sterilization Knowledge Center
What is E-Beam Sterilization?

Electron Beam sterilization uses a linear accelerator, which takes standard electricity and transforms it into high-speed, high-energy electrons. These electrons are then carefully directed using a magnetic field to create a precise curtain of energy. Carried on a conveyor, products pass through this curtain, where sterilization occurs.
What
Electron Beam (E-Beam) processing uses tightly controlled radiation to eliminate pathogens and other harmful microorganisms, helping to make products safe for use.
How
Electrons are accelerated to extremely high velocities in a state-of-the-art machine. When products are bombarded with these electrons, radiation is created throughout the product. This radiation eliminates harmful germs by disrupting their genetic material.
Why
E-Beam sterilization ensures product safety and is environmentally friendly: no lingering radiation or dangerous residues are created.
Electron Beam Sterilization Advantages
We use electron beam (E-Beam) accelerators as our radiation-generating technology. The benefits are clear.

Precise
E-Beam technology focuses high-energy electrons using a magnetic field. This controlled approach ensures targeted, consistent, and repeatable dosing to products, ensuring consistent and precise results.

Reliable
E-Beam is a well-understood technology. Developed initially in the 1950s, modern E-Beam accelerator systems are highly reliable industrial machines. We support our system, which is designed for > 95% uptime, with trained onsite technicians, remote support, and a large stock of replacement parts in case of component failure.

Sustainable
The E-Beam process takes clean electricity as input and produces radiation that is safely contained inside a small, controlled area. Radiation completely instantly when power is shut off. Electron Beam technology has a strong track record of safety: zero fatalities in 60+ years of operation.
Take our Electron Beam Suitability Quiz
Comparing Sterilization Modalities
Comparing sterilization modalities is essential to help make informed decisions about which sterilization method is most appropriate for specific products, taking into account factors such as effectiveness, material compatibility, processing time, cost, and environmental impact.| EtO | Gamma | X-ray | E-Beam | |
|---|---|---|---|---|
| Source | ||||
| Technology Maturity | High | High | Medium | High |
| Process quantity | Pallet | Boxes | Pallet | Boxes |
| Processing time | Days | Hours | Minutes | Seconds |
| Sustainability & Environmental impact | Toxic gas must be contained EPA legislating new limits now |
Synthetic radioisotopes are required for process Safety record is good but security risks are real |
As clean as the electricity used to power the system | As clean as the electricity used to power the system |
| Benefits | • Chemical sterilant with excellent absorption • Wide materials compatibility |
• Good penetration / tight Dose Uniformity Ratio (DUR) performance • Almost no power required. |
• Whole-pallet processing • Tight DUR performance |
• Extremely efficient: sustainably process most volume / year • Best $ / capacity available |
| Limitations | • Residuals problematic • Litigation risk • Environmental risk • New regulatory risk |
• Limited supply of Co-60 radioisotopes • New geopolitical sensitivity in wake of UKR war, China stance |
• Most technology risk (few sites operational) • Consumes most power |
• Products requiring tight DURs are challenging • Large / dense products can be challenging |
| Outlook | ❌ Slow phase-out over decades due to Environmental & litigation risk |
❌ No growth: isotope supply issues & geopolitical risk |
✅ Growth: Replace gamma for DUR-sensitive products |
✅ Growth: Efficient, sustainable technology |
Electron Beam Sterilization Frequently Asked Questions
Electron Beam (E-Beam) processing scans products with high-energy electrons, causing ionizing radiation to permeate the product. Most commonly this radiation is used to sterilize medical devices and other products, but other specialized material modification applications (crosslinking and chain scission) are also available.
E-Beam sterilization works by scanning products with high-energy electrons. To produce these high-energy electrons, a linear accelerator takes normal grid electricity and uses radio waves to accelerate electrons to ~99.9% of the speed of light. These electrons are “scanned” up and down by a magnetic field, creating a curtain of high-speed electrons through which products are moved via conveyor.
In sterilization applications, these high-energy electrons create ionizing radiation inside the product itself, which destroys contaminant microbes by breaking apart genetic material and other molecules that these microorganisms need to grow and multiply.
The irradiation/sterilization process occurs inside a large concrete bunker, which is designed and tested to harmlessly contain the radiation produced. An automated conveyor system carries products through the treatment area.
One of the most attractive benefits of the E-Beam process is that it can be the final step in producing sterile product: in most cases, complete cartons of products can be safely irradiated.
E-Beam sterilization ensures product safety and is environmentally friendly: no lingering radiation or dangerous residues are created. E-Beam does not carry the supply challenges associated with Gamma sterilization, nor the environmental or litigation risk associated with Ethylene Oxide (EO) sterilization. However, like EO and Gamma, E-Beam is regarded as a proven, “Established Category A” sterilization modality by the FDA. It is also highly scalable – modern E-Beam facilities process multiple truckloads of product each day.
E-Beam is very mature. The first linear accelerators were conceptualized and prototyped in the late 1920s. Johnson & Johnson introduced the first commercial E-Beam systems for medical use in 1956.
In the 1960s the technology matured, with different use cases explored in hospital radiotherapy, industrial processing, and materials science.
In the 1970s, advances in fundamental particle accelerator research in addition to modern computerized controls advanced the technology significantly in terms of total system power and reliability.
The industry has steadily produced higher energy / higher power machines with higher reliability levels. Today, machines like NextBeam’s reliably process multiple truckloads of customer products each day and benefit from exceptional reliability.
Radiation crosslinking is a process used to enhance the physical properties of polymers by creating covalent bonds between their molecular chains. This is achieved by exposing the polymer to high-energy radiation: in this case from an E-Beam system. The radiation generates free radicals (ions that are highly reactive/unstable) within the polymer structure, which then react to form crosslinks between the polymer chains, delivering improved material performance. Keep reading “E-Beam Crosslinking – A Basic Guide“.
NextBeam can help with this assessment. With some basic information about the product’s packaging configuration and dose requirements, we can quickly assess whether your product is E-Beam compatible and, if so, how efficiently it may be processed. At a minimum, testing will include dose mapping to ensure that the product can be processed within the required dose range. However – the dose mapping process is typically quick and low cost.
The microbiology testing is very similar to that performed for gamma sterilization and is comprised of bioburden recovery, bioburden enumeration, bacteriostasis/fungistasis, and a dose audit. In most cases it is possible to leverage historical testing for some of the microbiology tests. If testing is required, we can assist with defining the appropriate procedures, test methods, sample sizes, and acceptance criteria.
Regulatory activity for changing sterilizers or sterilization methods can vary based on the device type and where the device is marketed. In the United States, E-Beam is considered an “Established Category A” method, defined by a long history of safe and effective use in the industry, that may reduce or eliminate the need for regulatory notifications or submissions.
NextBeam can help with this assessment. With some information about the product materials, packaging configuration, and dose requirements, we can provide input about E-Beam as potential fit for your product. At a minimum, testing will include dose mapping to ensure that the product can be processed within the required dose range. If the product has not been qualified in radiation, max dose testing must also be carried out.
The microbiology testing is very similar to that performed for EO sterilization, comprised of bioburden recovery, bioburden enumeration, bacteriostasis/fungistasis, and fractional treatment to evaluate bioburden resistance. In most cases it is possible to leverage historical testing for some of the microbiology tests. If testing is required, we can assist with defining the appropriate procedures, test methods, sample sizes, and acceptance criteria.
Happily, EO sterilization validations are typically more complex that E-Beam validations: E-Beam validations do not require the validation of multiple process stages of preconditioning, gas exposure, and aeration. There are no lethal chemicals involved, and no need to test for residuals.
Regulatory activity for changing sterilizers or sterilization methods can vary based on the device type and where the device is marketed. In the United States, the FDA regulates these changes but has provided guidance
A typical timeline for an electron beam sterilization validation is about 8 weeks from the time that samples are final-packaged and ready for testing.
At NextBeam we recommended prioritizing dose mapping and max dose testing as a first step in a new E-Beam sterilization validation: these are low cost and relatively fast processes that can significantly de-risk the validation process early.
The most common method of sterilization validation is VDmax, which will typically use the following sample sizes:
- 3 lots of 10 – bioburden enumeration test
- 3 to 5 units – bioburden recovery
- 3 to 6 units – bacteriostasis / fungistasis
- 2 sets of 10 units – dose audit
A typical procedure for VDmax sterilization validation would work as follows:
- Irradiate the bioburden recovery and bacteriostasis samples. After irradiation, send these samples to the microbiology lab for bioburden recovery and B&F test, respectively.
- After bioburden recovery test is complete, test the 3 lots of 10 samples for bioburden enumeration.
- Using the bioburden enumeration data, determine the verification dose to be used for the dose audit from the tables in ISO 11137-2 (15 or 25 kGy) or ISO 13004 (17.5 – 35 kGy).
- Irradiate 10 devices for the dose audit, then send them to the microbiology lab for a test of sterility.
- Depending on the results of the dose audit, testing may be concluded, or it may be necessary to irradiate the second set of 10 dose audit samples.
Electron beam works best for low density materials up to about 0.2 grams per cubic centimeter. It may be possible to process higher density materials depending on the packaging configuration.
Subject to specific quality requirement constraints, NextBeam can help clients to optimize product configuration for maximally efficient E-Beam processing.
This depends on the product. For medical devices in the United States, quality requirements are defined by the Quality System Regulation (QSR), 21 CFR Part 820. Other quality systems can include ISO 13485 or ISO 9001.
The standards used for defining, validating, and routinely monitoring electron beam sterilization processes from a technical perspective include a wide variety of documents – based on what is being sterilized – including the ISO 11137 series and ASTM E61 documents.
Electron beam technology is extremely safe and environmentally responsible. As compared to gamma irradiation, there is no use or production of radioactive material, nor is there any risk of radiation escaping the concrete bunker. Additionally, unlike ethylene oxide sterilization, E-Beam technology does not require nor produce large quantities of toxic/carcinogenic chemicals.
You Might Be Wondering
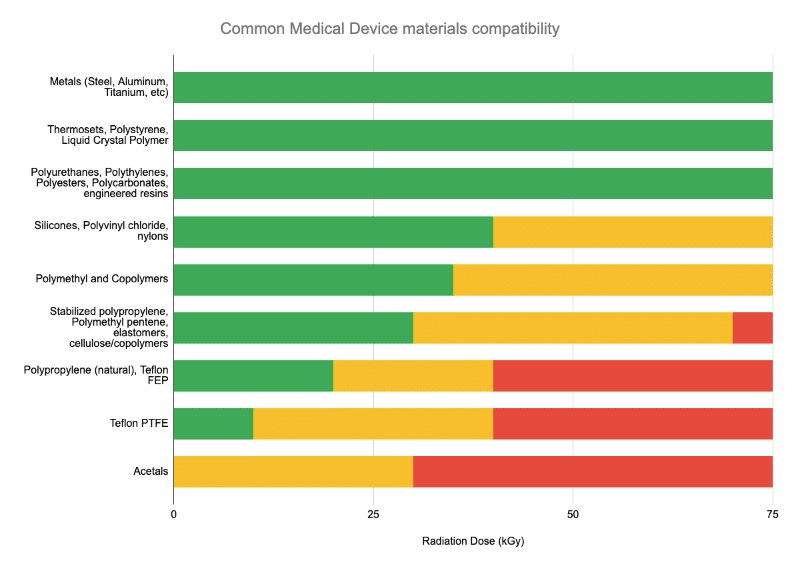
Is E-Beam Compatible With My Product?
Typically, if a device has previously been qualified in Gamma, E-Beam sterilization tends to be equivalent-to-gentler on the device due to E-Beam’s rapid delivery vs Gamma (seconds vs hours).
Which Industries Use E-Beam?
E-Beam sterilization is employed across various industries, including Medical Device, Laboratory Supply, Food & Consumer, Packaging and Pharma and Bioprocessing.
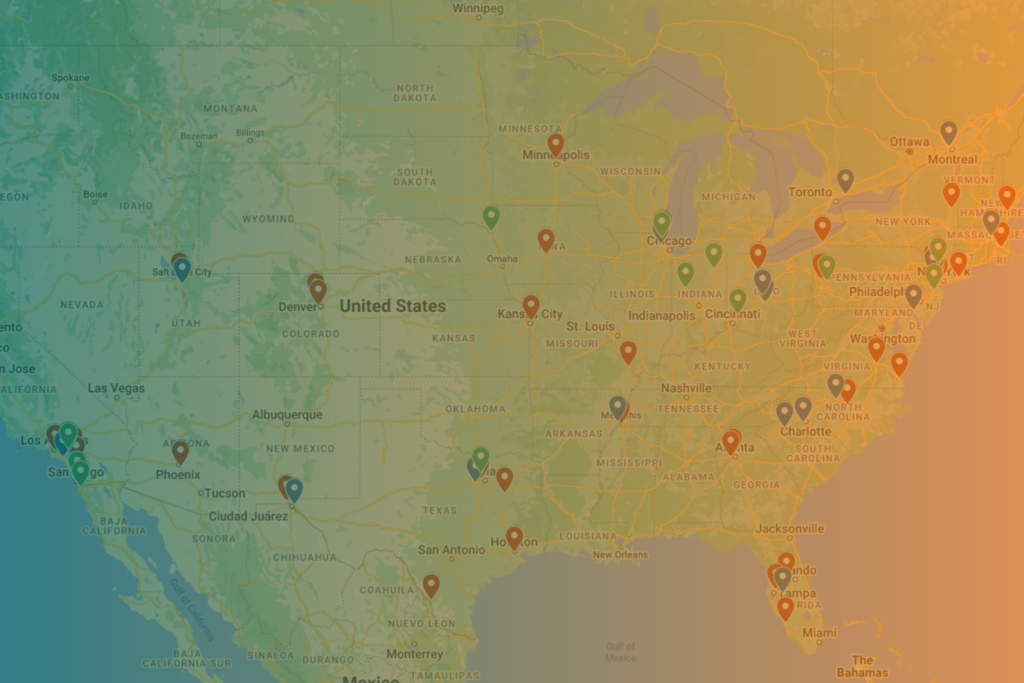
Where is a Sterilization Facility Near Me?
We understand the regional nature of sterilization: quick, convenient turnarounds are best for customers – and geography plays into this. Explore the sterilization map, created with publicly available data.
Your Sterilization Questions, Answered
Complete this form, or email hello@nextbeam.com, and a sterilization expert will get back to you within one business day.

All of our processes meet the highest-grade industry benchmarks for quality, designed from the ground up to adhere to ISO 13485, ISO 11137, and ISO 9001 standards for medical device manufacturing, radiation sterilization, and quality. We work across industries, from medical devices to laboratory science, pharmaceuticals, and others. Our collaborative approach is designed to deliver the right solutions for your sector. We process volumes ranging from a fraction of a pallet to multiple full truckloads of product.