November 2024 was an eventful month for Ethylene Oxide (EO) sterilizers:
- Cosmed announces bankruptcy filing – Reuters
- Steris discloses hundreds of ethylene oxide lawsuits over device sterilization plant – Medical Design and Outsourcing
It brings us no pleasure to write about these events. We have written about the “new normal” of EO litigation before. In short – we expect that EO litigation will continue, with harmful effects on the small-to-medium businesses in the EO space.
EO is not evil – but we need to recognize that we are living in a “new normal” in terms of litigation risk
EO is an incredibly high performance chemical sterilization technology – its penetration, lethality, and broad compatibility with typical medical device materials have made it a method of choice in sterilization for decades.
However, it is this highly penetrative lethality that makes EO difficult to operate in an environmentally-friendly way, and, historically, this has caused some level of concern over fugitive emissions being released into communities surrounding these facilities. Consequently we have seen major lawsuits filed against EO operators alleging that these EO emissions have caused elevated cancer rates in the communities that surround these facilities.
In the industry, there are those that question the validity of the science behind both these lawsuits and the new EPA regulations that will be going into effect over the coming several years.
At NextBeam, we are certainly not experts on the environmental safety of EO. But we do believe that the news of the past year underscores our view of the sector: EO has substantial litigation risk attached to it presently and this will continue into the future. How long will it be until the users of these facilities are drawn into litigation themselves? Hard to say.
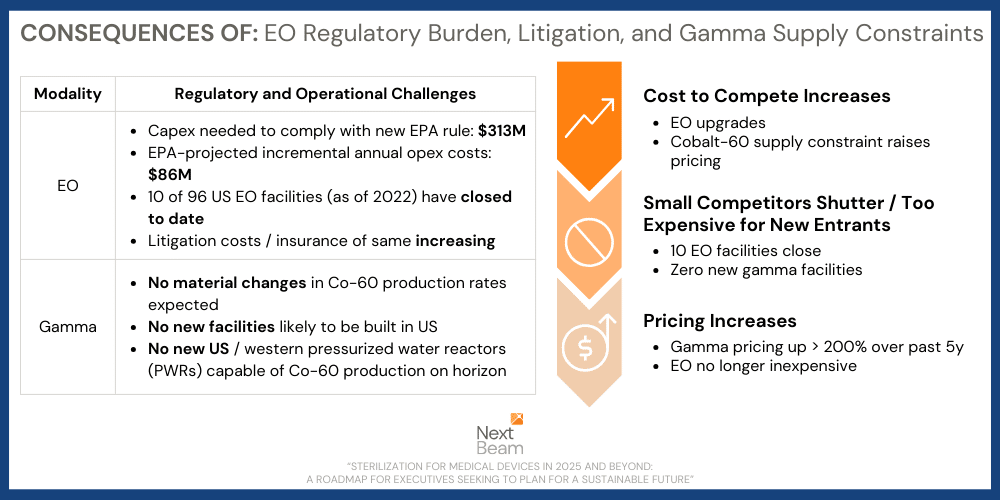
More EO regulations → higher EO prices → fewer service providers → even higher EO prices
Additionally, while the regulations promulgated by EPA earlier this year should help EO plants be operated with reduced emissions going forward, they will have consequences in the marketplace: the additional investment required to bring existing facilities into compliance with the new regulations is capital-intensive and will result in higher EO service pricing going forward.
And it doesn’t stop there. These facility investments are difficult enough for small/midsize EO providers to afford. But combine these with the ongoing litigation risk that being an EO provider now involves (e.g. soaring insurance premiums – if you can get insurance – and/or legal bills) and we have a recipe for seeing even stronger providers, like Cosmed, forced to file bankruptcy with their future operations in limbo.
So, who remains in business in situations such as these? The largest and most well-capitalized companies… which are typically the ultimate beneficiaries of increasing regulation. We expect the largest companies in the space to command only increased pricing power into the future as these combined effects of litigation and regulation continue to play out over the coming 5 years. The consequence? EO – once cheap and highly available – will no longer be so.
Accelerator-based sterilization can ride to the rescue
EO is 50% of the sterilization market, historically, because it is both highly compatible with most devices and inexpensive. As EO pricing increases, and in a world where Gamma capacity (e.g. Cobalt-60 supply) is unlikely to grow, accelerator-based sterilization is the only mature, sustainable, and scalable technology available to the industry.
Accelerator-based sterilization requires no scarce input, like Co-60, and does not pose the environmental risks of EO.
Migrating from EO to accelerator-based sterilization is feasible for many products
Depending on the product, the feasibility of migrating from EO to accelerator-based sterilization can range from being straightforward to not possible. But there are a host of tools, new technologies and regulatory pathways that are making these shifts easier than ever:
- Virtual dose mapping technologies – can be used to help users understand dose distribution in products in-silico and early in the development process
- Radiation-stable, PTFE-alternative coatings – the happy side-effect of a new generation of PFAS-free high-lubricity surface coatings is that they are significantly more radiation-compatible.
- TIR 104 and liberalization of rules for 510(k) – for 510(k) approved devices – FDA allows manufacturers to shift devices between any Established Category A modality – including from EO to radiation sterilization – with proper testing and documentation.
Breaking the vicious cycle
When we began the research that would lead us to found NextBeam, we didn’t set out to be an E-Beam provider. But market analysis, customer interviews, and research into the different modalities available led us to our view – that accelerator-based modalities are sustainable (environmentally and geopolitically), mature in the eyes of FDA and other regulators, and highly scalable.
It’s been clear for years now that EO is fraught with risk. We believe that it is high time to recognize that these risks are increasingly intolerable to the businesses and the communities in which they operate. We acknowledge that there are situations in which migrating away from EO is impractical. But industry growth, coupled with limited supply, means that we ought to be reserving the use of EO for when we absolutely must use it, rather than for reasons of convenience.
We will likely still be using EO in ten and possibly twenty years, for specialty products. But we should not let these exceptions stand in the way of a large-scale transition out of EO towards more sustainable sterilization technologies.
At NextBeam we are already working every day with customers, both large and small, who are taking decisive and significant steps to help future-proof their sterilization supply by transitioning to accelerator-based modalities. Please reach out to talk through the requirements of your specific product with us.
Andrew Patton
Founder/CEO
NextBeam
December 2024
Additional Articles We Think You Might Like
Have a question? Speak with a sterilization expert today, at your own convenience.