E-beam (electron beam) sterilization is a highly effective method for sterilizing medical devices and other products. Its characteristics make it ideal for specific types of products. Below we include a *non-exhaustive* list of example products that are ideal for E-Beam, both in terms of effectiveness and efficiency.
Examples:
- Surgical instruments (metal and many plastics / polymers)
- Syringes, needles, pipette tips, etc.
- Catheters (those that do not contain PTFE)
- IV sets, fittings, and accessories
- Wound care products (e.g., bandages, gauze)
- Small orthopedic devices (e.g. bone screws)
- Stents
2. Pharmaceuticals and Biologics
Examples:
- Single-use pharmaceutical containers and vials
- Certain active pharmaceutical ingredients (APIs)
- Biological implants (e.g., collagen matrices and other tissue products)
E-Beam is ideal for biologics and pharmaceuticals because they are typically small, lightweight, and have modest dose requirements. Additionally, there is no risk of leaving chemical residues, as there is with EO, and these products are sterilized in their final packaging, ensuring product integrity.
3. Packaging Materials
Examples:
- Plastic packaging (e.g., PET, PE, and PP)
- Blister packs
- Pouches and films for sterile barriers
- Food packaging systems (e.g. bags and fitments)
- Metallic foil or other non-vented packaging used for humidity sensitive products
Most modern packaging systems offer excellent radiation compatibility. E-Beam offers minimal risk of material degradation compared to gamma radiation in certain plastics, and the speed of processing for compatibly-sized devices is very high. For large amounts of packaging that are sterilized together and require more depth, X-ray is an ideal candidate for qualification.
4. Consumer Products
Examples:
- Baby products like pacifiers, bottles, and nipples
- Cosmetics packaging
- Menstrual products
- Personal care items (toothbrushes, razors)
E-Beam is ideal for these lightweight products because it is a simple and very fast way of assuring a target sterility level is achieved. Additionally, these products typically require very low doses, which tends to help accelerate their processing speed. Not having to consider the removal of EO residuals is a further plus.
5. Food and Agricultural Products
Examples:
- Dry food items (e.g., spices, herbs).
- Fresh produce (for microbial reduction).
- Animal feed and veterinary products.
E-Beam is highly suitable for products where chemical sterilization is undesirable due to the possibility of dangerous residuals and/or deleterious effects of steam or thermal sterilization.
For lower-density food products packaged in smaller containers (e.g. < 0.2g/cc with depth dimensions less than 40cm), E-Beam can be highly effective and efficient. The challenge for food and agricultural products typically is density and size: double-sided E-Beam cannot penetrate much more than 8cm of water (e.g. 1 g/cm3 products like meats). For these high-density products, it may be possible to configure the packaging configuration for at least one thin dimension, or consider using a different technology such as X-ray.
5. Laboratory and Research Products
Examples:
- Lab equipment like pipettes and petri dishes.
- Single-use consumables (e.g., test tubes, filters).
E-Beam offers laboratory and research products many of the same benefits that it does for medical devices: highly reliable and efficient sterilization of final-packaged devices without the complications of residuals.
Key Advantages for Ideal Products
For all products, E-Beam’s key advantages can be summed up as follows:
Fast, Scalable, and Economic
- E-Beam is very high throughput; sterilization can typically be completed in seconds for individual products. E-Beam is also a highly-serial process, meaning that it is highly scalable to truckload-sized jobs, increasing cost-efficiency.
Low-risk
- Environmental / litigation: E-Beam offers no environmental risk and thus no risk of lawsuits.
- Supply chain: E-Beam requires grid power to operate – no hard-to-source radioactive materials are needed, and none are produced, meaning that there is no ongoing risk to supply
- Maturity: E-Beam is long recognized by FDA and similar global regulatory agencies as being as mature as all other technologies (“FDA Established Category A”) – there is no regulatory risk.
Gentlest Process for Radiation-Compatible Materials
- Residuals: E-Beam does not leave chemical residues, making it ideal for sensitive applications where residuals are dangerous or their absence is difficult to confirm.
- Dry Process: Does not involve moisture, making it suitable for dry materials and some electronics.
- Non-Thermal: it is a non-thermal process and appropriate for devices that would be damaged by heat.
- Material Compatibility: Works well with a variety of radiation-compatible polymers, metals, and other materials.
- Minimal Radiation-Degradation (if any): as compared to Gamma and X-ray, E-Beam is gentlest on materials, as its speed of dose delivery is highest, minimizing dwell time in a highly oxidative environment
Considerations
While e-beam sterilization is suitable for many products, it has important limitations:
- E-Beam is not ideal for products with high-density materials or large dimensions that cannot be efficiently subdivided into sizes that E-Beam may efficiently process, as the penetration depth of the high-energy electrons created by an E-Beam accelerator is not as high as photon-based processes such as Gamma and X-ray. For large, dense pallet loads of materials, these technologies are superior.
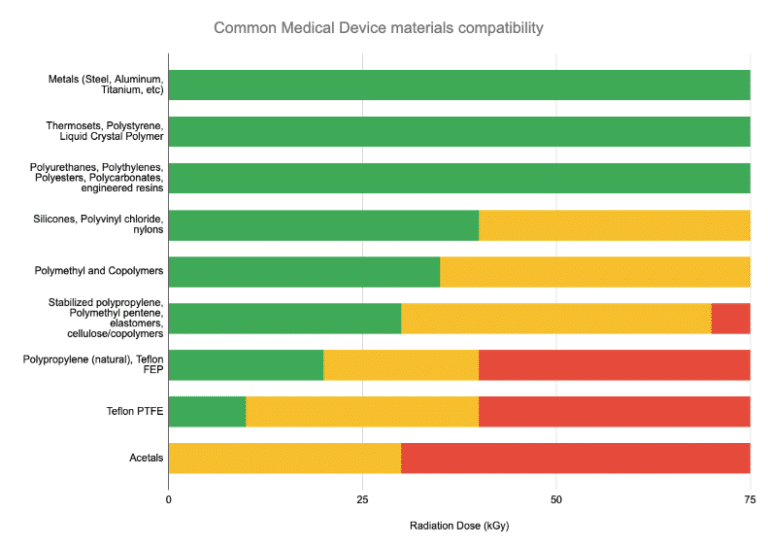
- While many polymers and materials are highly radiation-compatible, not all are. Certain sensitive materials (e.g., some polymers or electronics) may degrade in radiation. Fortunately, up-front testing can be rapidly and inexpensively done to assure manufacturers that their devices are compatible with E-Beam (and, for that matter) any other radiation technology.
Manufacturers should always assess the compatibility of their own specific products with e-beam sterilization through testing and validation. At NextBeam we work with customers across industries every day to help them understand the benefits and risks of E-Beam processing. Call us today for a free consultation.
Additional Articles We Think You Might Like
Have a question? Speak with a sterilization expert today, at your own convenience.