Industries We Serve
Want to work with us?
Contact Us
→ Medical Device
→ Laboratory Supply
→ Food & Consumer
→ Packaging
→ Pharmaceutical & Bioprocessing
→ Pet Food & Treat Safety
Take our quiz to understand if you’re a good fit for Electron Beam sterilization →
Medical Device Sterilization
Industries We Serve
Want to work with us?
Contact Us
→ Medical Device
→ Laboratory Supply
→ Food & Consumer
→ Packaging
→ Pharmaceutical & Bioprocessing
→ Pet Food & Treat Safety
Take our quiz to understand if you’re a good fit for Electron Beam sterilization →
Medical Device Applications
Leverage E-Beam’s best possible material compatibility and FDA-recognized maturity to safeguard your products.
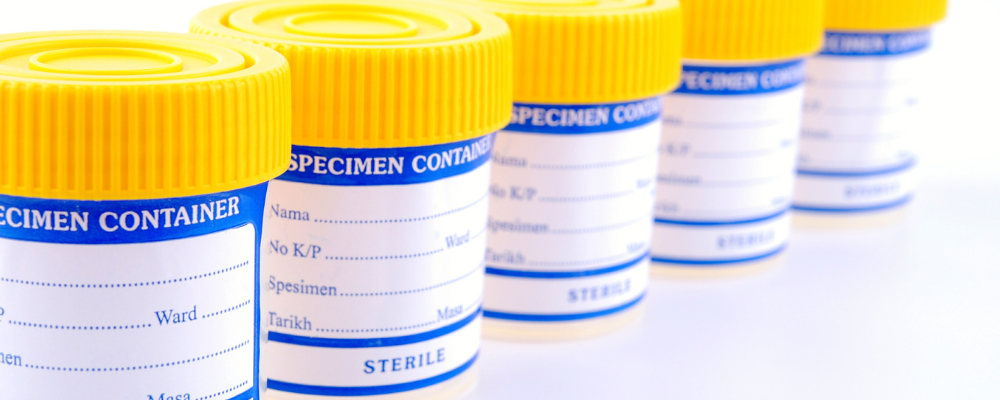
High-Volume Single-Use Devices
- Rapidly sterilize truckloads of high-volume single use devices: from sterile cotton swabs to specimen collection cups, E-Beam is a highly reliable and efficient method for large volume sterilization
- Eliminate longterm concerns around risks present with legacy modalities: litigation risk for EO and escalating costs for both EO and Gamma processing.
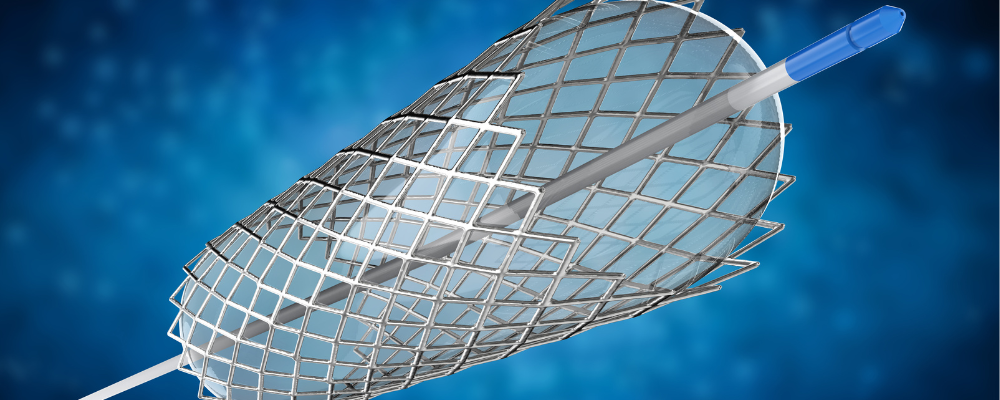
Highly-Engineered Devices
- For lower-volume, high-value devices such as implantable medical devices, E-Beam’s precision, reliability, and rapid dose delivery assure that the customer can achieve the best possible sterilization outcome.
- Eliminate concerns around complex qualification processes (e.g. EO residuals) and future-proof expensive products by designing for E-Beam upfront.
Why MedTech Industry Leaders Are Choosing E-Beam
Precise and Efficient
E-Beam provides precise irradiation in seconds, leaving no harmful chemical residuals. Our $20M facility processes multiple truckloads daily, ensuring fast distribution.
Enhanced Material Compatibility
E-Beam’s quick dose delivery minimizes material degradation, making it ideal for sterilizing radiation-compatible medical devices without prolonged oxidative exposure.
Sustainable & Safe
E-Beam sterilization uses conventional power, offering a safer and greener alternative to Gamma and Ethylene Oxide, without carcinogenic or radioactive materials.
Reliable Results
Our ISO-certified process delivers precise, large-scale sterilization, preserving the quality of medical devices without heat or extended radiation exposure.

Well-Located & Logistically Convenient
NextBeam provides contract sterilization for the following major midwestern metro areas:
📍 Chicago, IL
📍 Minneapolis, MN
📍 Denver, CO
📍 St. Louis, MO
📍 Kansas City, MO
Our central location ensures efficient logistics, rapid turnaround times, and convenient access for businesses of all sizes.
Subscribe to our newsletter to learn about future expansions.
Leverage medical device-grade quality for your business
Our quality system is fully compliant with ISO 13485, ISO 11137, and ISO 9001 for medical device manufacturing, radiation sterilization, and quality management systems.












Let's connect
Complete this form, or email hello@nextbeam.com, and a sterilization expert will get back to you within one business day.
FAQs

- Precise and reliable dose delivery assures the integrity and safety of medical devices.
- Efficient E-Beam technology helps deliver highest quality yet cost-effective outcomes.
- NextBeam meets or exceeds rigorous ISO 13485, ISO 11137, and ISO 9001 measures.
- Individual products usually only need a few seconds of precisely calculated irradiation, making processing bulk products and large shipments highly efficient. Once irradiated, products are immediately safe for handling: E-Beam leaves no trace of chemicals or radiation.
- NextBeam’s state-of-the-art facility is designed for high throughput and typically processes product in under 5 days. Expedite options can be made available.



