You may have heard the terms “surface dosing” and “dose mapping” around the industry and might be wondering where these come from. The “what” here is straightforward:
- Surface Dose Processing – A process where the irradiator treats the product as a “black box” and applies radiation to a surface of the product only. What happens inside the product is not measured; there is no qualification required.
- Dose-Mapped Processing – A process that involves a product-specific qualification step where theory and experimentation are used to determine what happens inside a product: where the maximum and minimum dose of radiation occur and what these relative values are during an irradiation process. Once this qualification process is complete, the production irradiation process is defined in a process specification.
Your Industry Matters
For customers in industries with highly regulated products – most famously the medical device industry – there is rarely any choice here – their products must be fully qualified via dose mapping.
But there are other industries with less stringent or no regulatory requirements – where customers know that surface dosing will improve the quality of their product, even if the detailed information isn’t known.
And further – for max dose testing, proof-of-concept feasibility, and other specific testing that regulated customers engage in, surface dosing can be an effective approach for coupon testing and other activities.
So how does it work? When is surface dosing appropriate to use vs. performing a dose map?
What You Get From Dose Mapping?

For almost any sterilization process, whether radiation, heat, steam, or even chemical sterilants, “too much” is a bad thing. Yet each process comes with its own variation.
When it comes to radiation sterilization, the proper grilling of a thick steak provides a good analogy: The middle of the steak cannot be undercooked, while the outside of the steak should not be incinerated. In the same way, with radiation sterilization, we are seeking to provide the minimum (e.g. required for sterilization) dose while staying below the maximum dose (e.g. dose above which the product may be damaged).
The dose mapping process is the qualification step by which we determine where the minimum and maximum (think “hot or cold”) points are in the product, and what the ratio of dose is between the two.
E-Beam Theory Proves Helpful
The average E-Beam dose deposition through a material is well-characterized, with the graph pictured below for a constant-density product under irradiation:
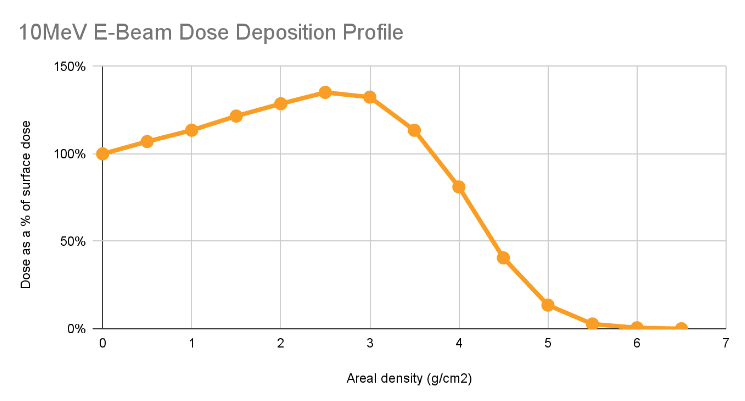
As can be seen in the graph, the dose is greater than or equal to surface dose until reaching approximately 3.5 g/cm2.
Said another way, for single-sided irradiation, if the beam is irradiating less than 3.5 g of mass under each square centimeter of the face of the product, we expect that the minimum dose experienced will occur at the face of the product. Therefore, even though we do not know the details of exactly how the product absorbs dose, we would expect that it will be at least the dose applied to the surface of the product.
Double-sided processing can offer some relief from the 3.5g/cm2 limit, but often will require some level of dose mapping – particularly in regulated product. Contact us for more detail.
When is Surface Dose Acceptable or Suitable For the Purpose of a Test?
(These are whitelist examples: if any of these are true then dose mapping is not required)
- If the product/material was shot through a dimension where the areal density is under 3.5 g/cm2, and the maximum dose is not practically relevant (e.g. the material is not sensitive to any sort of practical overdose, surface dosing can be acceptable).
- For testing, where the target dose of the test is the min dose on the product (e.g. max dose testing), if the surface dose is the min dose then there is no more information to gain from dose mapping.
- If the dose to a product doesn’t have any quality or regulatory requirement where the dose distribution within the test sample is required to be known, surface dosing is sufficient. We run into some customers who have no requirement on the dose: they know from experience that treating the product with radiation improves its function, and they are not seeking to make any specific claims about the product (e.g. sterility) that would require dose mapping as part of the process.
When is Surface Dose Not Realistic Or Allowed?
(These are blacklist examples: if all of these are true then dose mapping is required)
- Most obviously, if both the min and max dose have to be measured for the purpose of the test, surface dosing is not appropriate.
- Treating multiple boxes of multiple materials or overlapping units: without a dose map there is no way to assure that a minimum dose is delivered.
- If the combination of product / material / purpose of the test has some regulatory / quality requirement that requires the dose distribution to be known, surface dosing is inappropriate.
Surface Dosing Can Be a Useful Tool — You Just Need to Understand What You’re Getting
At NextBeam we wholeheartedly encourage our customers to make pragmatic decisions that save them time and cost – but only if they have clarity on what they do and do not get. Surface dosing is a process that we will happily run for our customers seeking to get quick feedback on the right kind of coupon, max dose, and other feasibility testing.
We can help walk you through what this means for your specific product and application. Contact us today.