You could switch to electron beam in a matter of days.
At NextBeam we often speak with medical device manufacturers that labor under a common misconception: transferring a gamma sterilized product to electron beam (E-Beam) requires expensive and time-consuming technical and regulatory work, and ultimately requires FDA approval.
Thankfully, this is not the case for the vast majority of devices!
Switch to E-Beam in a Matter of Days
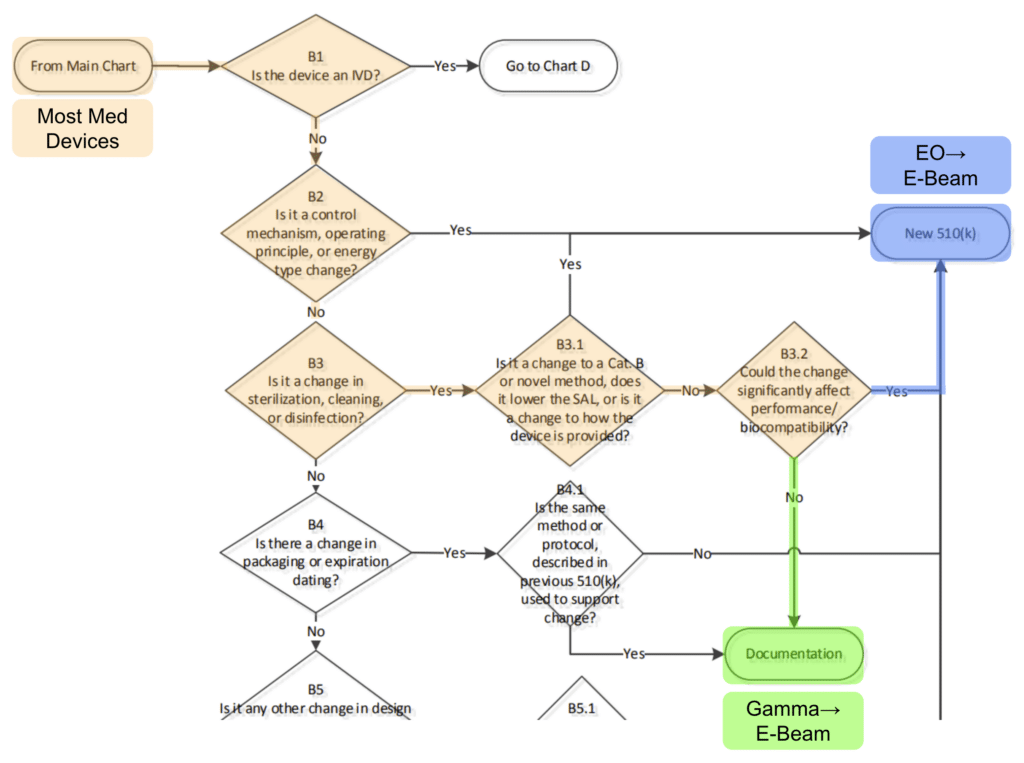
Following the flowchart from the FDA’s published guidance on “Deciding When to Submit a New 510(k) for a Change to an Existing Device” it is possible for many device types to transfer from gamma to e-beam in a matter of days with dose mapping and internal documentation.
It’s true! Most device manufacturers can readily take advantage of the superior availability and lower costs of E-Beam over gamma sterilization in just a few days work and total re-qualification costs of just a few thousand dollars.
Why Medical Device Manufacturers Are Switching from Gamma to Electron Beam Sterilization
- Lower cost
- More availability capacity (current and planned)
- Same, familiar validation tests and sample sizes following ISO 11137-2
- Reduced supply chain risk
- Transferring between radiation technologies is fast and easy for most devices
- Higher dose rate of e-beam is gentler on most device and packaging materials, resulting in superior materials compatibility
What About Devices Sold in Europe?
You might be thinking, ‘but the FDA only covers devices sold in the USA… would this be a significant change under EU MDR Article 120(3)?’
Several notified bodies for CE marking have also clarified in presentations and guidance documents that changing from gamma to e-beam or x-ray would not by itself be considered a significant change as long as there are no secondary effects such as a change in device materials performance or biocompatibility that would introduce new or greater risk, similar to the position described in FDA guidance.
Curious about the process? Connect with a NextBeam sterilization expert.
Additional Articles We Think You Might Like
Have a question? Speak with a sterilization expert today, at your own convenience.