November 22, 2022
It’s been a busy first several weeks of production here at NextBeam as we rapidly qualify customer products.
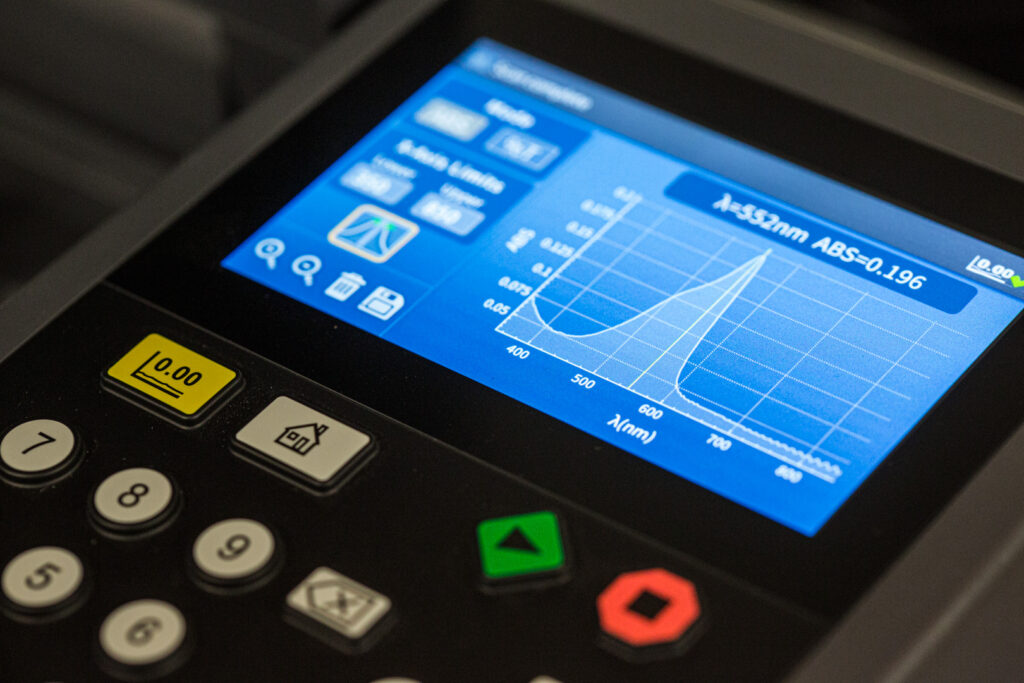
Our highly-skilled team uses a state-of-the-art dosimetry laboratory to conduct dose mapping accurately and rapidly. Additionally, we use modern, cloud-based tools to analyze data, create dose map reports, and route these reports for review/approval on tight timelines.
The resulting performance is clear: 50 products fully qualified for volume production within 71 business days — an average of less than 2 days to qualify each product.
Compare this to what we hear from customers: they are regularly quoted anywhere from 8-12 weeks to qualify the product!

Additional Articles We Think You Might Like
Have a question? Speak with a sterilization expert today, at your own convenience.