A Familiar (and Often Simpler) Pathway
Like EO and Gamma, E-Beam has its own unique aspects that should be considered when developing a sterilization validation for a new product. This article offers a rough outline of what you can expect for a from-scratch E-Beam validation project.
If you’re familiar with Gamma validations, this process will be very similar: like Gamma, the industry-recognized process for E-Beam sterilization is described in the ISO 11137 standard for Sterilization of Healthcare Products: Radiation.
While the standard is focused on healthcare products, this same approach to validation can be applied across non-medical industries (such as labware, packaging, etc.) and product types as a thorough, experimental approach to constructing a product-specific process for either sterilization or bioburden reduction.
When Can I Start Validation?
Validating sterility is not just validation of the product itself – it includes validation of the entire manufacturing and packaging process as well. So, an ISO-compliant validation can only be completed when the manufacturing and packaging processes are defined and ready to support low-volume production.
However, planning for sterilization early in the product development lifecycle (e.g. prior to manufacturing and packaging processes being ready) has always been good practice.
Now, however, it’s critical: especially for radiation-compatible products where E-Beam is a candidate modality. Very simple adjustments to product, packaging, and manufacturing design can be tremendously impactful on how easy the product is to sterilize. The good news is that there are computational tools available to help engineers design for sterilization. These tools, combined with rapid feasibility testing at an E-Beam provider like NextBeam, can help reduce the risk that new product development may unwittingly create a product that is nearly impossible to sterilize at scale.
Validation Answers 3 Key Questions
In radiation sterilization, the phrase “dose is dose” is sometimes used to illustrate that the type of radiation produced is not of primary importance: it is the dose that is delivered throughout the product. So, whether gamma, E-Beam, or X-ray, radiation sterilization validation is focused on determining the same 3 things:
- What is the minimum dose? Select and validate a minimum dose of radiation required to ensure that the product is sterilized to the sterility assurance level (SAL) desired.
- What is the maximum dose? Demonstrate a maximum dose that the product can be subject to without degrading it or impairing its function.
- Do min and max doses fall within the achievable min and max? Validate that the product may be processed in a production configuration such that the dose will remain between these lower and upper bounds.
The Sequence We Recommend
While any radiation sterilization process involves the same components, E-Beam differs from X-ray and Gamma modalities in that it is much more sensitive to dose mapping.
As in Gamma (ISO 11137) and other (eg. Ethylene Oxide / ISO 11135) validations, the validation process involves the customer (manufacturer), sterilizer (in this case irradiator), and a microbiology laboratory.
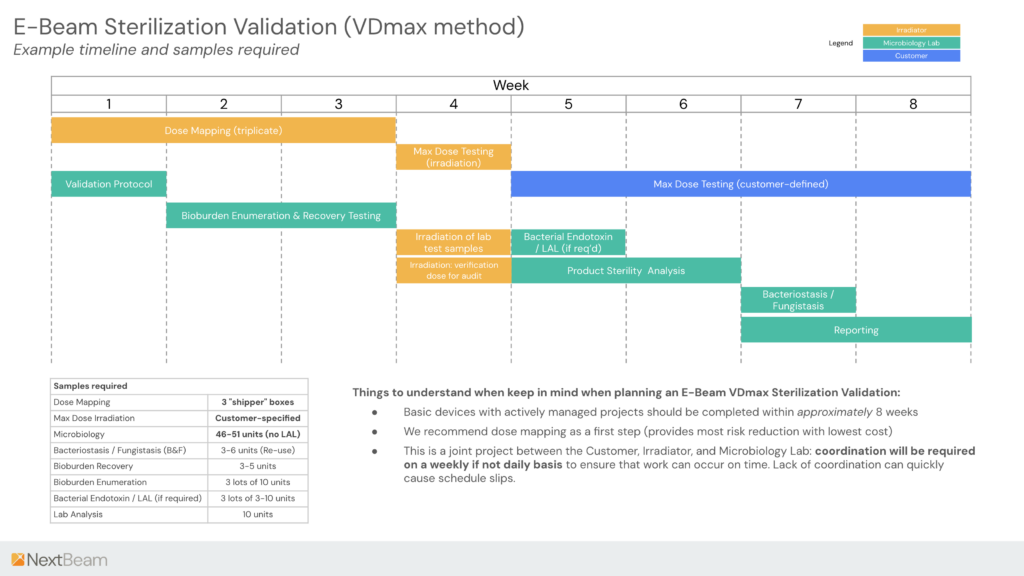
We lay out a rough timeline for the sterilization process below.
As the timeline demonstrates, a tightly managed sterilization validation process can be completed in around 8 weeks.
Roles and Responsibilities
The Customer’s role involves:
- Provide production-representative samples
- Being the subject matter expert on the function of the product – understanding any unique risks to sterilization (e.g. difficult-to-expose areas) as well as how to evaluate the product’s functionality after sterilization
- Project manage the validation process – though this can be delegated in some cases to third-party firms that offer this as a service.
The Irradiator’s role comprises:
- Providing irradiation services in accordance with ISO 11137 (and ISO 13485, for regulated medical devices)
- Evaluating the product-specific requirement for both dose mapping and max dose testing services
- Executing test irradiation runs as well as triplicate dose mapping and reporting
The microbiology lab’s role involves:
- Developing a protocol for sterilization validation
- Designing and executing microbiology lab tests
- Analyzing resultant data and reporting on this information.
Detailing Out the Process Elements
Dose mapping is typically straightforward and can be completed quickly for most products. No information about the dosing required for sterility or the maximum acceptable dose is necessary: the dose mapping process only seeks to determine the ratio of the maximum dose that the product experiences to the minimum dose experienced – thus it can be run independently of understanding the sterilization dose or the max allowable dose.
Dose mapping “for “credit” according to the ISO standard is carried out in triplicate (to capture a minimal amount of statistical variation across products).
Microbiology Validation may be carried out in parallel with dose mapping. Depending on the strategy selected to establish the sterilization dose, a wide range of product quantity may be required to complete this step, though the VDmax method typically consumes < 100 individual units. Regardless of the strategy used, the endpoint is the same: a validated minimum dose is required to assure sterility.
The slate of microbiology testing specified includes testing designed to quantify the relative cleanliness of the product (determine bioburden), as well as detect the possible presence of harmful endotoxins or bacteriostatic or fungistatic elements (all of these elements introduce more complexity and risk into the process if detected). Tests include:
- Bioburden enumeration – a test to count the number of colony-forming units (CFUs) of bacteria on test samples. Per ISO 11137, bioburden is a critical parameter for determining the amount of radiation required to acheive a target SAL.
- Bioburden recovery – a test to validate the effectivity of the bioburden enumeration test method (sterilize a device, introduce a known amount of bacteria, then validate that a representative amount of bacteria are collected using the enumeration technique.
- Bacterial Endotoxin / LAL – a critical assay used to detect and quantify bacterial endotoxins in pharmaceuticals, medical devices, and other products (typically only required for implantable / surgical devices).
- Bacteriostasis / Fungistasis – a test used to detect any antimicrobial properties that would inhibit the growth of microorganisms during sterility testing. This is crucial to ensure that any contaminating organisms present in the sample are detected.
- Product Sterility Analysis – for VDmax this involves dosing products with partial sterilization doses to confirm that the statistical results support that achievement of a certain SAL.
How Much Product Do I Need in Order to Complete a VDmax Validation?
There can be a wide range of time and cost deltas depending on the complexity of the device.
Typically, the following amount of product units is required for a VDmax validation:
| Validation Area | Samples Required |
|---|---|
| Dose Mapping | 3 "shipper" boxes |
| Max Dose Irradiation | Customer-specified |
| Microbiology | 52-57 units total |
| Bacteriostasis / Fungistasis (B&F) | 3-6 units (Re-use) |
| Bioburden Recovery | 3-5 units |
| Bioburden Enumeration | 3 lots of 10 units |
| Bacterial Endotoxin / LAL (if req’d) | 3 lots of 3-10 units |
| Product Sterility Analysis | 10 units |
Should I Hire an Outside Consultant to Run a Validation?
If you’re not familiar with the process, there are expert consultants that can either consult on the validation, delivering specific elements, such as a written protocol, and/or manage the full process with minimal oversight required.
Outsourcing can make sense, depending on your organization’s level of technical expertise, available manpower. Contact NextBeam for recommendations on which service providers may be most helpful to you.
Additional Articles We Think You Might Like
Have a question? Speak with a sterilization expert today, at your own convenience.