E-Beam and Gamma Irradiation Background
The first commercial E-Beam sterilization processes were introduced by Ethicon (now part of J&J) nearly 70 years ago. But at the time, E-Beam was not powerful or reliable enough to scale well, and the nuclear industry was ascendant (this is a world before Chernobyl and Three Mile Island).
Gamma was, and remains, a high-performance technology: great DUR performance with virtually zero power requirement. The cobalt-60 Gamma relies on could be produced easily in the pressurized heavy water reactors (PHWRs) that were being installed and scaled up decades ago.
This worked well for a long time. Gamma’s market dominance (40% of today’s sterilization market – the largest radiation sterilization modality by processing volume) is a testament to that. But the continual supply constraints/price increases that customers face for lack of Co-60 supply today, in addition to the lack of new Co-60 producing reactors coming online within the next decade suggests that the nuclear power industry is unlikely to support much medical device industry sterilization growth in the next decade.
The Gamma Hangover
While E-Beam is not new, it has come a long way over the past decade in terms of the available processing throughput and reliability it offers customers.
Still, the physical penetration profile of a 10MeV electron is limited. For most medical devices, relatively low density is not a challenge. But so many years of Gamma being a dominant technology has caused most radiation-compatible devices to be qualified for Gamma – as that was what made sense originally. Anyone who has worked in device design or engineering also realizes the tendency to use “the last thing that worked” as the baseline for the new device if there is no reason for change.
Enter X-Ray
Accelerator manufacturers have long wanted both great penetration and freedom from Gamma’s Co-60 supply constraints. X-ray technology (see diagram below) offers this, though at significant cost: the physics of the conversion mechanism are highly inefficient, and consequently, the system must be significantly (5-10x) more powerful to deliver the same throughput.
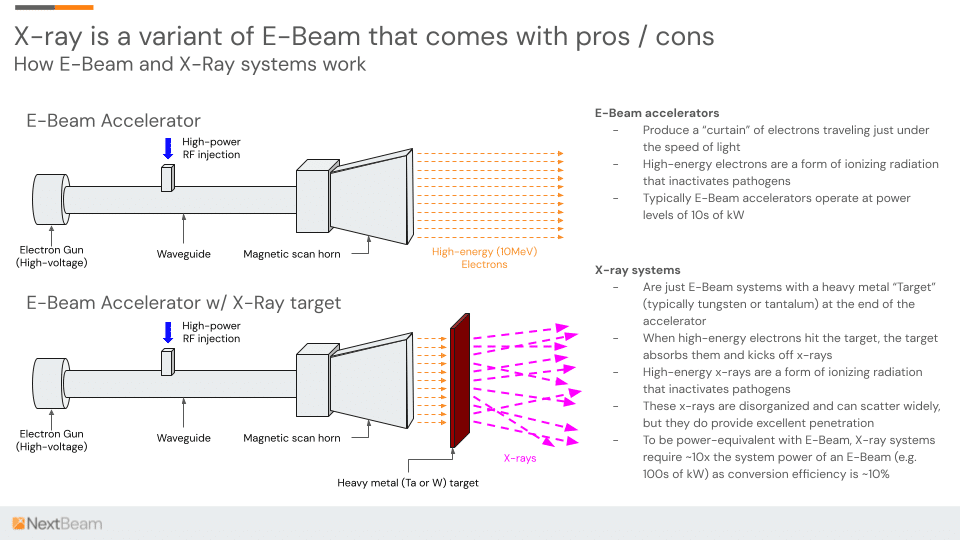
In sum, the massive (~90%) power loss during the E-Beam-to-X-ray conversion process means that high power X-rays can consume around a megawatt of power – 10x the requirement of a comparable-throughput E-Beam. The capital cost of the X-ray facility is likely to be 2-3x. All this adds up to pricing that is almost guaranteed to be consistently higher: the need for Co-60 may have disappeared, but the pricing increases associated with Gamma have not.
Do I Need X-Ray?
We sometimes hear from prospective customers that “need X-ray.” While some, in fact, do have products that require the deeper penetration profile of X-ray, we find disconcertingly often that most believe they need X-ray simply because it matches a gamma dose distribution profile. This is often a misconception: products can be processed more rapidly and inexpensively in E-Beam delivering a significantly better experience for said customers. Sometimes the shipper-box level packaging of the product might need to be reconfigured to support E-Beam, but for many medical devices these changes are relatively simple.
Additionally, X-ray is still relatively rare: at the time of this writing (April 2024), there are no in-production high-power X-ray contract sterilization facilities online in the US. But they are coming: we are aware of at least 4 contract sterilization X-ray facilities in development whose rollout has been significantly delayed.
How Technology Drives The Economics Of Sterilization
When technologies with very high capital and operating costs become dominant in markets, they tend to become less – not more – competitive. Over time, this means that end users pay more for outcomes as opposed to less. Lower-cost technologies tend to be more positively disruptive in that they allow for more competition with corresponding benefits to customers.
Our NextBeam view on X-ray is that it is a great technology and we look forward to its continued maturation. But we are on a mission to deliver as much sustainable sterilization capacity as we can. In terms of maximizing the value of our capital and operating spend, E-Beam delivers far more capacity per dollar, which we can then pass on to customers in the form of better pricing.
A Hybrid Approach
We believe that a portfolio of accelerator-based modalities should be dominated by E-Beam with the ability to run X-ray when (and if) necessary. We estimate that the split between modalities should be 80/20 E-Beam/X-ray.
E-Beam works wonders for low-density, low-dose products like syringes, pipette tips, etc. X-ray can be useful for items where penetration is needed (e.g. large orthopedic implants, sterile saline solution, etc).
Breaking Old Habits
Decades of Gamma being the dominant mode of radiation sterilization has conditioned the industry to design products for Gamma sterilization. Many Gamma validations typically miss what can be “easy” opportunities to set E-Beam-friendly requirements:
- Shipper Box-Level Design Misses: Most “shipper level” boxes contain multiple SKUs with individual sterile barriers that are packaged together for transport. 40 years ago, In a world of dominant Gamma, there was no need to package products in a way that made it easy for E-Beam to process. Reconfiguring these shipper boxes such that they have more consistent geometry (eg products held in place effectively inside) and/or have one dimension shrunk such that there is less mass for an E-Beam to penetrate is a low-tech and straightforward process.
- Low Max Dose: All too often we see devices with sterilization doses of 25kGy qualified for a max dose of 40kGy. The relatively low max dose that the product is qualified to is an artificial limit in the sense that it was all that the developers *bothered* to qualify at the time – not the true max dose the device could sustain. Re-examining / testing for higher max dose can also deliver the flexibility that E-Beam needs for processing.
We see easy, long-term wins for sterilization customers in expanding “Design for Manufacturing” (DfM) concepts to include “Design for Sterilization” and improving on the above opportunities. We are already working with large customers who are seeking to make their supply chains more robust and sustainable.
Breaking Old Habits
Decades of Gamma being the dominant mode of radiation sterilization has conditioned the industry to design products for Gamma sterilization. Many Gamma validations typically miss what can be “easy” opportunities to set E-Beam-friendly requirements:
- Shipper Box-Level Design Misses: Most “shipper level” boxes contain multiple SKUs with individual sterile barriers that are packaged together for transport. 40 years ago, In a world of dominant Gamma, there was no need to package products in a way that made it easy for E-Beam to process. Reconfiguring these shipper boxes such that they have more consistent geometry (eg products held in place effectively inside) and/or have one dimension shrunk such that there is less mass for an E-Beam to penetrate is a low-tech and straightforward process.
- Low Max Dose: All too often we see devices with sterilization doses of 25kGy qualified for a max dose of 40kGy. The relatively low max dose that the product is qualified to is an artificial limit in the sense that it was all that the developers *bothered* to qualify at the time – not the true max dose the device could sustain. Re-examining / testing for higher max dose can also deliver the flexibility that E-Beam needs for processing.
We see easy, long-term wins for sterilization customers in expanding “Design for Manufacturing” (DfM) concepts to include “Design for Sterilization” and improving on the above opportunities. We are already working with large customers who are seeking to make their supply chains more robust and sustainable.
How We Can Help
At NextBeam we are always happy to provide free consultation and rapid feasibility testing to help quickly and inexpensively provide the data and context needed to support evaluations of E-Beam for customers.
Additional Articles We Think You Might Like
Have a question? Speak with a sterilization expert today, at your own convenience.






