Planning for Sterilization
Sterility is crucial for ensuring the safety and effectiveness of medical devices. For many decades, the sterilization market offered a number of simple choices. But, like so many other industries, massive growth and new constraints are changing the way medical device manufacturers consider sterilization in their product development and manufacturing process.
Engineers and purchasing professionals have options when considering what type of sterilization to use for their medical devices. In this note we provide a list of technical and business factors for consideration as well as an overview of the most common methods used for volume sterilization of single use medical devices.
Key Items to Consider
When comparing and contrasting different sterilization modalities, key technical and logistical factors should be considered to make an informed decision.
Technical Factors
- Sterilization Effectiveness: The sterilization method must be able to effectively kill all microorganisms on the medical device. Certain types of devices may not be conducive to certain modalities of sterilization – e.g. products that are not able to be reliably permeated by toxic gas, for instance, or are too dense to be acceptably penetrated by radiation.
- Compatibility with the Medical Device: Different sterilization modalities may have varying effects on the materials used in the medical device. It is important to ensure that the sterilization method selected does not damage the device in a way that materially degrades its efficacy or marketability.
- Safety: Safety is an important consideration in the selection of a sterilization method. Depending on the interaction between the device and modality, toxic residues may be left on the device.
- Regulatory Requirements: Different sterilization methods may be subject to different regulatory requirements, which should be considered when selecting a method.
Logistical Factors
- Cycle Time: The time required for sterilization can vary greatly between different modalities. This can impact the overall manufacturing process and time-to-market for the device, and, depending on the nature of the product and the level of cleanliness of the facility, certain methods may take too long to be practically employed.
- Cost: The cost of sterilization can also vary greatly depending on the modality selected. The cost of each modality can vary significantly depending on the nature of the product itself, thus, there is not necessarily a consistent “cheapest option” when it comes to selecting a modality.
- Geographic Availability: Depending on the final assembly location of the product to be sterilized, some modalities may not be easily/cost-effectively accessible in these areas.
- Future Outlook: Some modalities face supply challenges due to environmental risk, geopolitical risk, and litigation risk. It is important to understand the outlook and expectation of modality supply when developing or managing a medical device product.
Technical Resources to Consider:
Comparing Sterilization Modalities
Comparing sterilization modalities is essential to help make informed decisions about which sterilization method is most appropriate for specific products, taking into account factors such as effectiveness, material compatibility, processing time, cost, and environmental impact.| EtO | Gamma | X-ray | E-Beam | |
|---|---|---|---|---|
| Source | ||||
| Technology Maturity | High | High | Medium | High |
| Process quantity | Pallet | Boxes | Pallet | Boxes |
| Processing time | Days | Hours | Minutes | Seconds |
| Sustainability & Environmental impact | Toxic gas must be contained EPA legislating new limits now |
Synthetic radioisotopes are required for process Safety record is good but security risks are real |
As clean as the electricity used to power the system | As clean as the electricity used to power the system |
| Benefits | • Chemical sterilant with excellent absorption • Wide materials compatibility |
• Good penetration / tight Dose Uniformity Ratio (DUR) performance • Almost no power required. |
• Whole-pallet processing • Tight DUR performance |
• Extremely efficient: sustainably process most volume / year • Best $ / capacity available |
| Limitations | • Residuals problematic • Litigation risk • Environmental risk • New regulatory risk |
• Limited supply of Co-60 radioisotopes • New geopolitical sensitivity in wake of UKR war, China stance |
• Most technology risk (few sites operational) • Consumes most power |
• Products requiring tight DURs are challenging • Large / dense products can be challenging |
| Outlook | ❌ Slow phase-out over decades due to Environmental & litigation risk |
❌ No growth: isotope supply issues & geopolitical risk |
✅ Growth: Replace gamma for DUR-sensitive products |
✅ Growth: Efficient, sustainable technology |
Ethylene Oxide Sterilization

Ethylene Oxide Sterilization Background
Developed for sterilization purposes in the 1930s, Ethylene oxide (EO) is a gas that is highly effective at killing microorganisms on medical devices. It is commonly used for the sterilization of heat-sensitive devices such as plastic, rubber, and electronics. Ethylene oxide is highly effective and can penetrate suitably-designed packaging materials to reach all areas of the device. However, it is highly flammable and explosive, and its use requires specialized equipment and facilities. EO also requires a lengthy aeration process to remove residual gas from the device before it can be used.
Ethylene Oxide Sterilization Pros
EO is attractive because it is historically low-cost and offers superior material compatibility and can safely sterilize devices made of materials that are not radiation-compatible (e.g. teflon, certain fabrics, etc.). EO also can work at the pallet level, meaning that there is no requirement to depalletize / repalletize.
Ethylene Oxide Sterilization Cons
Sustainability is a challenge for EO. It has also been classified as a human carcinogen by the EPA, has been the subject of nearly $1 billion of ongoing legal action, and occasionally has explosive and catastrophic failures.
Ethylene Oxide Sterilization Outlook
We expect that regulatory scrutiny will continue to increase, and consequently, additional EO capacity in North America will be limited if not flat. For all of the reasons stated above, we do not consider EO a sustainable modality for sterilization.
Gamma Radiation Sterilization
Gamma Radiation Background
This method uses high-energy gamma rays to kill microorganisms on the medical device. It is effective, fast, and does not require any special handling of the device. However, it can cause material degradation over time, and the equipment required can be expensive.
Gamma dates from the late 1950s, when the first facility was built in Stuttgart, Germany, and used to process spices. In 1963, the first gamma irradiator was installed in the US for medical device sterilization.
Gamma’s key ingredient is the highly radioactive cobalt-60 isotope, which is produced when small amounts of cobalt are placed inside a nuclear reactor for extended periods of time. The resulting isotope is then removed and carefully transported in rad-shielded containers to a facility that is designed to safely contain the isotope and expose it to target products as needed.
Gamma Radiation Pros
Gamma is attractive because the radiation produced by cobalt-60 is highly penetrative and (generally) able to process an entire pallet at a time with highly uniform dose distributed throughout (e.g. low dose uniformity ratio, or DUR).
Gamma Radiation Cons
Supply is a challenge for Gamma. There are a limited number of nuclear plants around the world capable of producing Co-60 and approximately 1/3rd of these are located in geopolitically challenging countries (Russia and China). While the operation of gamma sites has traditionally been very safe, the National Nuclear Security Agency (NNSA) has an ongoing program to help reduce North American gamma consumption to reduce risk of loss of highly radioactive material.
Gamma Radiation Outlook
We expect gamma to remain in place across North America, but supply challenges to effectively constrain its growth to near zero over time.
Electron Beam (E-Beam) Sterilization
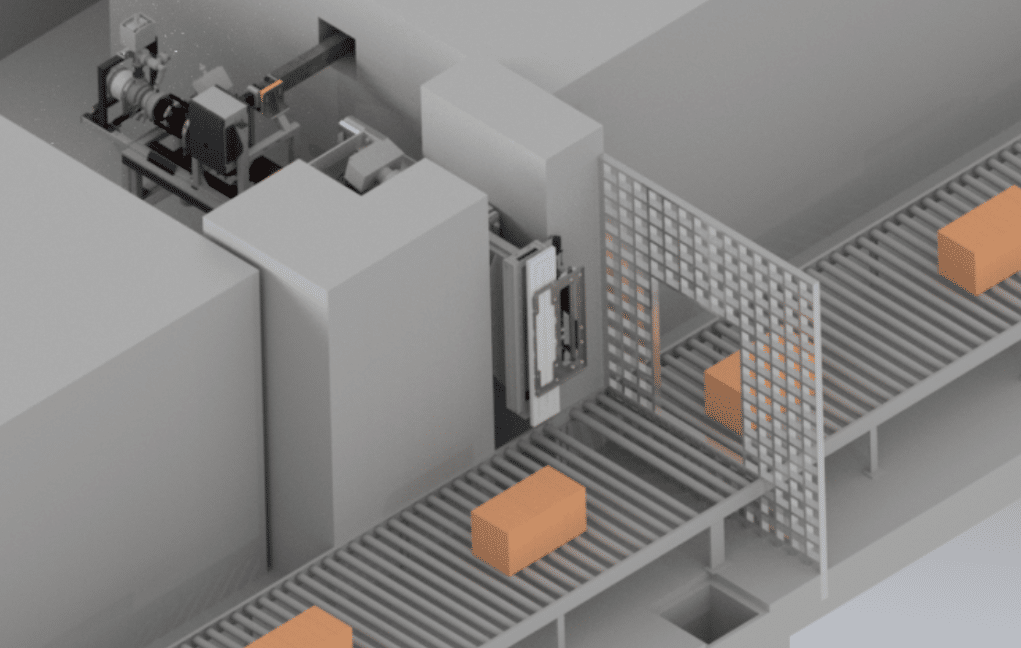
E-Beam Background
E-beam sterilization uses high-energy electrons to kill microorganisms on medical devices. The electrons penetrate the material and disrupt the DNA of any microorganisms present. Pioneered originally by Johnson & Johnson in the 1950s, E-Beam systems take grid power and transform it into very high-speed electrons that move in excess of 98% the speed of light. When they crash into atoms comprising a medical device and its packaging, radiation is produced, and this radiation is a sterilizing agent, killing bacteria and inactivating other pathogens.
E-Beam Pros
E-Beam sterilization is highly effective and can be used to sterilize a wide range of materials. It is also a relatively fast process (dose is typically delivered within seconds) and does not require the use of any chemicals. It is environmentally friendly, producing no dangerous emissions and neither requiring nor producing any radioactive materials.
E-Beam Cons
E-Beam requires the designing and construction of expensive specialized equipment and facilities. As with gamma, material compatibility can be a challenge. Finally, E-Beam systems offer lower penetration / higher DURs than gamma is capable of, making it generally unable to process full pallets of goods at once.
E-Beam Outlook
We view E-Beam as a mature and sustainable technology that can be integrated into any community without fear of nuclear risk or environmental consequence. We expect the medical device industry to strongly increase the amount of sterilization performed in E-Beam over the coming decade to avoid the litigation and supply limitations inherent to EO and gamma.
X-Ray Sterilization
X-Ray Background
X-ray sterilization is a relatively new technology that uses high-energy x-rays to kill microorganisms on medical devices. Effectively an application of E-Beam technology, X-ray uses a very large E-Beam accelerator but shoots the resulting electrons at a heavy metal target (typically tantalum or tungsten) where, on impact, they generate x-rays. The very low level of efficiency (~10% of E-Beam power) meant that volume X-ray sterilization was not practical until commercial linear accelerators > 100kW became available. Commercial X-ray efforts date from the 1990s, and as of early 2023 there are no operating facilities in North America, although several are under construction.
X-Ray Pros
X-rays are highly penetrative and can be used to sterilize a wide range of materials. Thus it is an attractive and obvious alternative to products that currently get irradiated in gamma sterilization. X-ray sterilization effectively combines the sustainability of E-Beam with the penetration/DUR of gamma.
X-Ray Cons
X-ray systems are extremely power-inefficient, which means that the linacs that power them must be 10x the size of a comparably powered E-Beam system. Additionally, they are relatively new in market and longterm reliability and performance have not yet been well-demonstrated.
X-Ray Outlook
We expect X-ray capacity to grow significantly over the next 10 years. The considerably higher facility capex as well as higher power costs will, however, make this a premium modality that will be used in instances when E-Beam technology is not suitable.
Changing Modalities: Gamma to E-Beam
Transitioning a medical device from gamma radiation sterilization to e-beam sterilization can be a complex process that requires careful planning and execution to ensure that the device is effectively sterilized and meets all regulatory requirements.
However, industry standards groups have been working to help define best practices to reduce the uncertainty around making these transitions. First published in 2022, AAMI TIR 104 offers guidance on how to transfer medical devices between gamma, electron beam, and X-ray sterilization modalities. Guidance includes what criteria need to be assessed or tested based on the product materials and the type of transfer (e.g. gamma to/from e-beam to/from to x-ray).
More on TIR 104:
- Who does it apply to? Any organization looking to transfer medical devices from one radiation modality to another (gamma, E-beam, X-ray) or from one site to another (E-beam to E-beam)
- What is it? AAMI TIR 104 provides guidance on how to transfer medical devices between gamma, electron beam, and X-ray sterilization. Guidance includes what criteria need to be assessed or tested based on the product materials and the type of transfer (e.g. gamma to/from e-beam to/from to x-ray).
- Why is it important for the industry? In recent years sterilization modalities like EO and gamma have become increasingly expensive, difficult to source, and environmentally unfriendly. Device manufacturers have been seeking ways to switch modalities while minimizing time and cost.
- How does it work? With TIR 104, industry experts have created a set of pragmatic recommendations that will help medical devices transfer between sterilization modalities with more speed and less cost than was previously possible. For compatible products, TIR 104 guides medical device companies to rapidly and cost-effectively generate quality datasets to demonstrate their products are compatible with other sterilization modalities, like E-Beam and X-ray.
- How will it change things? Rather than completely restarting a sterilization process, sterile device manufacturers now have a rapid and concrete path to qualify their products in a new modality, unlocking the door to more favorable sterilization options.
Which Products Are The Easiest to Transition?
In general, transitioning from Gamma to E-Beam can be simpler for products that on balance:
- Have wider acceptable dose uniformity ratios (DURs). Thin / light packaged products can work with low acceptable DURs, but as product thickness / density increases, higher DURs better accommodate E-Beam’s less penetrative nature.
- Cartons an areal density of less than 9 g/cm2 on at least one dimension
- Product is not unusually heat-sensitive – E-Beam delivers dose very quickly – often in seconds – and this causes product temperatures to rise more than a Gamma process.
The Transition Path
How do you determine if a sterile product is a good candidate for a TIR 104 transfer from gamma to E-Beam sterilization? Questions to determine if a product is a good candidate for transferring from gamma to E-Beam:
- Can the product be irradiated at the required min/max dose range at the candidate facility?
- What tests need to be done?
- Dose mapping?
- Microbiology testing?
- Materials / packaging testing?
- What documentation is required?
- Description of product and packaging materials and configuration
- Description of current and future irradiator
- Assess if microbicidal efficacy is affected (usually no for most products)
- Assess if product and packaging are affected by different dose rate (usually no for most products)
- Description of which doses are being transferred (sterilization dose, max dose, verification dose)
At NextBeam, we frequently help medical device manufacturer “refugees” from the Gamma sterilization world seeking to re-qualify their products in E-Beam.
We’re happy to help provide guidance and qualification services for those looking to leverage the benefits of TIR 104. Contact us to kick off a discussion.
Additional Articles We Think You Might Like
Have a question? Speak with a sterilization expert today, at your own convenience.








