What do I need to know in order to get a decent fidelity answer, quickly?
E-Beam processing has been around for a long time and is considered equally as mature (“Established Category A”) as Gamma sterilization by the FDA. However, we acknowledge that E-Beam qualification is different from, and less simple, than Gamma irradiation (it is also in many ways simpler than EO qualification, which is a topic for another article).
Understanding whether your product can be sterilized/processed in E-Beam involves understanding 2 major concepts:
- Material Compatibility
- Dose Distribution Requirements
At NextBeam, we developed an Electron Beam Suitability Calculator to help step through some of these questions. We’ve also outlined a checklist of information (below) needed to help make an 80% confidence level evaluation.
Material Compatibility
The easy upfront question here is: “Is your device already qualified in Gamma sterilization?”
If the answer is yes, then E-Beam should be equivalent or better in terms of material compatibility.
If your device has not yet been through any type of radiation sterilization qualification, understanding whether it contains any of the following polymer materials is helpful to get a first-pass sense of feasibility:
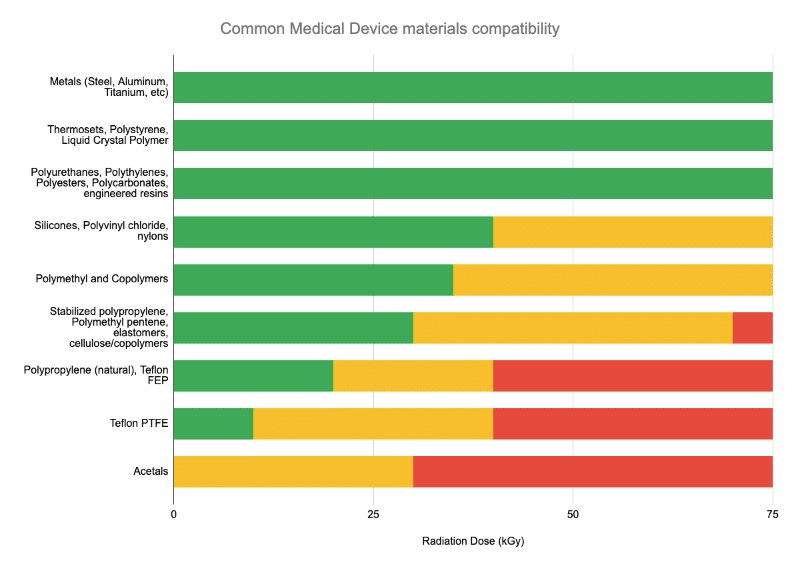
As can be seen in the chart, the presence of polymers itself is not disqualifying! In fact, most polymers are tolerant to high-radiation environments. However, some of the more common soft plastics with surface performance requirements (e.g. PTFE/Teflon) can be challenged in their radiation compatibility.
Understanding the materials in your product is important. To help read the table above: in general, most medical device sterilization doses are 25kGy or less. AAMI’s excellent TIR 17 is a detailed resource that can help users understand the performance of different materials.
Dose Distribution Requirements
While they are both forms of radiation sterilization, the pattern of dose distribution is where E-Beam and Gamma can differ greatly. While Gamma’s photons are highly penetrative and dose falls off relatively gently with increasing depth, the E-Beam depth-dose curve is far shallower. Still, E-Beam typically offers more than adequate penetration capability for most medical devices. Further, demonstrating this is easy: the E-Beam dose mapping process is quick and low-cost (<$3,000 and 5 days from receipt of samples) for most products. Contact us to get started.
As a first step, customers and irradiators prefer to use simple models to evaluate the areal density of the product that can help rapidly evaluate the likelihood of final-packaged product compatibility with E-Beam before any testing is executed. To explore these we typically look for 6 quantities:
- Minimum product dimension
- Median product dimension
- Minimum product dimension
- Mass of product
- Minimum dose required
- Maximum dose allowed
Having this information will help us rapidly analyze your product, or you can self-serve with the E-Beam Suitability Calculator and we’ll call you to talk through the results. 🙂
As always, at NextBeam we’re here to help answer questions about E-Beam sterilization. Contact us for a free consultation on your specific product needs.
Additional Articles We Think You Might Like
Have a question? Speak with a sterilization expert today, at your own convenience.





