We submit that there’s a smoother, faster way to structure and execute E-Beam sterilization validations.
How did we get here?
Traditionally, Gamma dominated the radiation sterilization market (90%+ of products were done in gamma). A typical gamma sterilization process flow is laid out below:
This approach makes a lot of sense for gamma, where penetration and depth-dose performance is excellent. But, with Cobalt-60 supply constraints and years of attendant price increases, device OEMs are looking for more sustainable and economical approaches to sterilization: E-Beam delivers these benefits. But while E-Beam is a superior technology from a sustainability and pricing perspective, it is disadvantaged in depth-dose penetration performance versus Gamma.
Addressing the Elephant in the Room, First
Given that we know the depth-dose performance of E-Beam is limited, it makes sense to address this early on in the validation process. There are two paths available to rapidly evaluate what dose range that a product will experience:
- Virtual dose mapping (VDM) tools – we have written before on the use of these advanced computational tools for running Monte Carlo simulations that project dose. We love VDM as a technology because it can help OEMs understand dose distribution very early on in the development process – before even prototype product is manufactured.
- Early physical dose mapping – the ISO 11137 standard for radiation sterilization still requires a physical dose map. But moving this forward in the process makes great sense for several reasons:
- Dose mapping is typically inexpensive (~$2,500 for a triplicate map) and quick (done in a few weeks, with faster turnaround times available).
- Dose mapping early provides a clear target for what maximum permissible dose should be, helping to clarify the maximum permissible testing process.
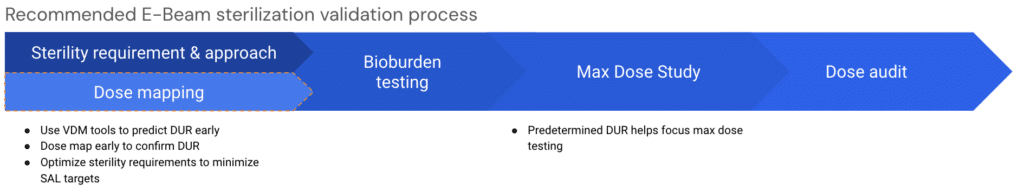
So, we think the sequence for a more efficient sterilization validation approach for E-Beam should look something like this:
The optimized (blue) pathway highlights the delays from the legacy (red). We think that this modification can help customers better understand how E-Beam will work with their product early, enabling them to run a more efficient and effective sterilization validation process.
NextBeam Is Here to Help
As always, please reach out to us at NextBeam for a free consultation with a sterilization expert – we’re happy to help. Contact us today to get started.
Additional Articles We Think You Might Like
Have a question? Speak with a sterilization expert today, at your own convenience.