
Compare E-Beam and Conveyor Configurations
Ready to optimize your product’s sterilization? Contact us today for a complimentary evaluation. Our sterilization expertise ensures consistent processing tailored

Electron Beam sterilization uses a linear accelerator, which takes standard electricity and transforms it into high-speed, high-energy electrons. These electrons are then carefully directed using a magnetic field to create a precise curtain of energy. Carried on a conveyor, products pass through this curtain, where sterilization occurs.
What
Electron Beam (E-Beam) processing uses tightly controlled radiation to eliminate pathogens and other harmful microorganisms, helping to make products safe for use.
How
Electrons are accelerated to extremely high velocities in a state-of-the-art machine. When products are bombarded with these electrons, radiation is created throughout the product. This radiation eliminates harmful germs by disrupting their genetic material.
Why
E-Beam sterilization ensures product safety and is environmentally friendly: no lingering radiation or dangerous residues are created.
We use electron beam (E-Beam) accelerators as our radiation-generating technology. The benefits are clear.

Precise
E-Beam technology focuses high-energy electrons using a magnetic field. This controlled approach ensures targeted, consistent, and repeatable dosing to products, ensuring consistent and precise results.

Reliable
E-Beam is a well-understood technology. Developed initially in the 1950s, modern E-Beam accelerator systems are highly reliable industrial machines. We support our system, which is designed for > 95% uptime, with trained onsite technicians, remote support, and a large stock of replacement parts in case of component failure.

Sustainable
The E-Beam process takes clean electricity as input and produces radiation that is safely contained inside a small, controlled area. Radiation completely instantly when power is shut off. Electron Beam technology has a strong track record of safety: zero fatalities in 60+ years of operation.
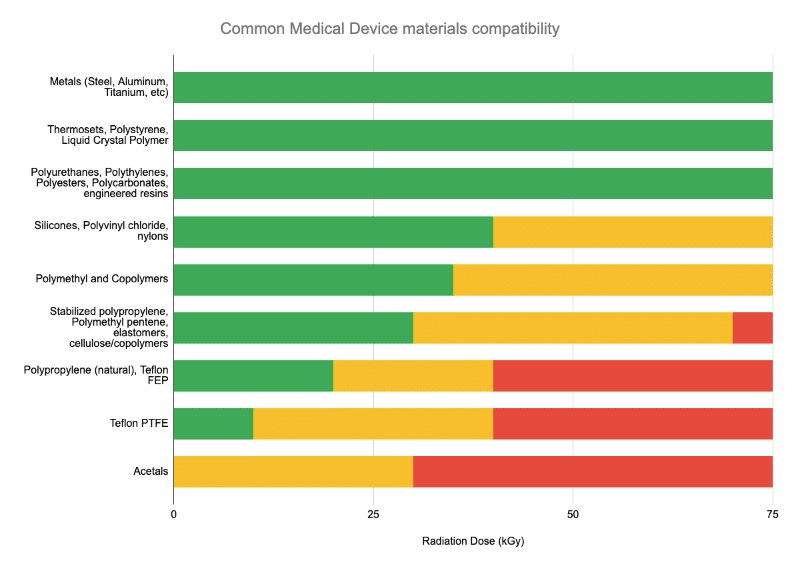
Typically, if a device has previously been qualified in Gamma, E-Beam sterilization tends to be equivalent-to-gentler on the device due to E-Beam’s rapid delivery vs Gamma (seconds vs hours).
E-Beam sterilization is employed across various industries, including Medical Device, Laboratory Supply, Food & Consumer, Packaging and Pharma and Bioprocessing.
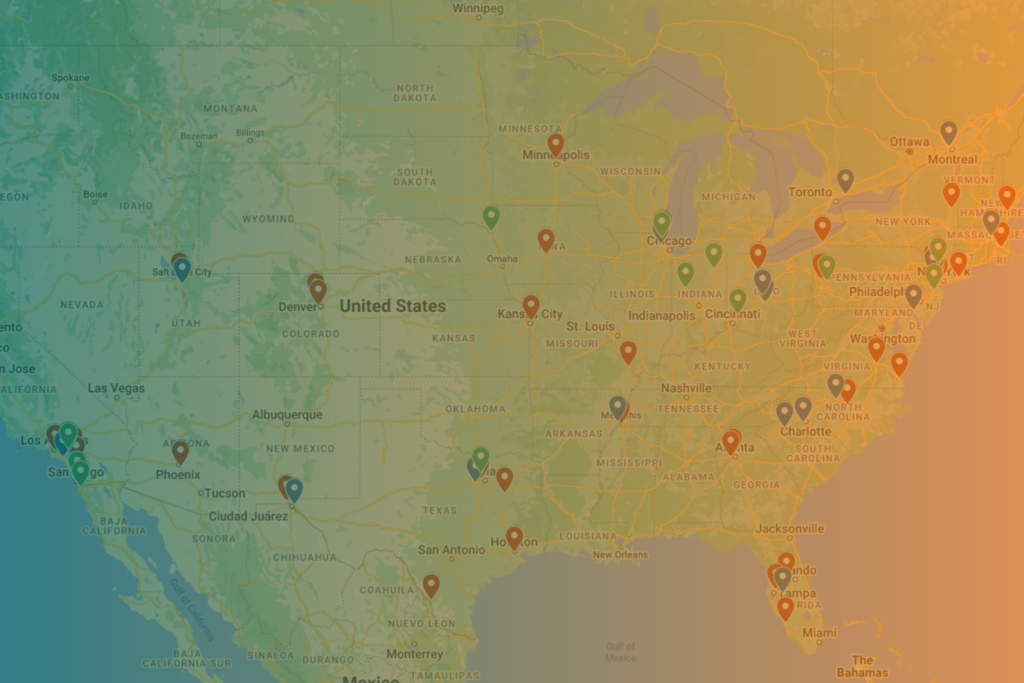
We understand the regional nature of sterilization: quick, convenient turnarounds are best for customers – and geography plays into this. Explore the sterilization map, created with publicly available data.
Electron Beam (E-Beam) processing uses tightly controlled radiation to eliminate pathogens and other harmful microorganisms, helping to make products safe for use.
The simple answer is that electrons are accelerated to extremely high velocities in a state-of-the-art machine. When products are bombarded with these electrons, radiation is created throughout the product. This radiation eliminates harmful germs by disrupting their genetic material.
Electron beam (“E-Beam”) irradiation works by scanning products with high-energy electrons. To produce high-energy electrons, a linear accelerator takes normal grid electricity and uses radio waves to accelerate electrons to nearly the speed of light. These electrons are “scanned” up and down by a magnetic field, creating a curtain of high-speed electrons through which products are moved via conveyor.
In sterilization applications, these high-energy electrons create radiation inside the product itself, which destroys bacteria, viruses, and other contaminants by breaking apart genetic material and other molecules that microorganisms need to grow and multiply.
The irradiation/sterilization process occurs inside a large concrete bunker, which is designed and tested to contain the radiation produced.. An automated conveyor system carries products through the treatment area.
One of the most attractive benefits of the E-Beam process is that it can be the final step in producing sterile product: in most cases, complete cartons of products can be safely irradiated.
E-Beam sterilization ensures product safety and is environmentally friendly: no lingering radiation or dangerous residues are created.
The first linear accelerators were conceptualized and prototyped in the late 1920s. Johnson & Johnson introduced the first commercial E-Beam systems for medical use in 1956.
In the 1960s the technology matured, with different use cases explored in hospital radiotherapy, industrial processing, and materials science.
In the 1970s, advances in fundamental particle accelerator research in addition to modern computerized controls advanced the technology significantly in terms of total system power and reliability.
The industry has steadily produced higher energy / higher power machines with higher reliability levels. Today, machines like NextBeam’s reliably process multiple truckloads of customer products each day and benefit from exceptional reliability.
NextBeam can help with this assessment. With some basic information about the product materials, packaging configuration, and dose requirements, we can provide input about E-Beam as potential fit for your product. At a minimum, testing will include dose mapping to ensure that the product can be processed within the required dose range.
The microbiology testing is very similar to that performed for gamma sterilization and is comprised of bioburden recovery, bioburden enumeration, bacteriostasis/fungistasis, and a dose audit. In most cases it is possible to leverage historical testing for some of the microbiology tests. If testing is required, we can assist with defining the appropriate procedures, test methods, sample sizes, and acceptance criteria.
Regulatory activity for changing sterilizers or sterilization methods can vary based on the device type and where the device is marketed. In the United States, electron beam is considered an “Established Category A” method, defined by a long history of safe and effective use in the industry, that may reduce or eliminate the need for regulatory notifications or submissions.
NextBeam can help with this assessment. With some information about the product materials, packaging configuration, and dose requirements, we can provide input about E-Beam as potential fit for your product. At a minimum, testing will include dose mapping to ensure that the product can be processed within the required dose range.
The microbiology testing is very similar to that performed for EO sterilization, comprised of bioburden recovery, bioburden enumeration, bacteriostasis/fungistasis, and fractional treatment to evaluate bioburden resistance. In most cases it is possible to leverage historical testing for some of the microbiology tests. If testing is required, we can assist with defining the appropriate procedures, test methods, sample sizes, and acceptance criteria.
Regulatory activity for changing sterilizers or sterilization methods can vary based on the device type and where the device is marketed. In the United States, electron beam is considered an “Established Category A” method, defined by a long history of safe and effective use in the industry, that may reduce or eliminate the need for regulatory notifications or submissions.
A typical timeline for an electron beam sterilization validation is about 6 weeks from the time that samples are final-packaged and ready for testing. The most common method of sterilization validation is VDmax, which will typically use the following sample sizes:
A typical procedure for VDmax sterilization validation would work as follows:
Electron beam works best for low density materials up to about 0.20 grams per cubic centimeter. It may be possible to process higher density materials depending on the packaging configuration.
Subject to specific quality requirement constraints, NextBeam can help clients to optimize product configuration for maximally efficient E-Beam processing.
This depends on the product. For medical devices in the United States, quality requirements are defined by the Quality System Regulation (QSR), 21 CFR Part 820. Other quality systems can include ISO 13485 or ISO 9001.
The standards used for defining, validating, and routinely monitoring electron beam sterilization processes from a technical perspective include a wide variety of documents – based on what is being sterilized – including the ISO 11137 series and ASTM E61 documents.
Electron beam technology is extremely safe and environmentally responsible. As compared to gamma irradiation, there is no use or production of radioactive material, nor is there any risk of radiation escaping the concrete bunker. Additionally, unlike ethylene oxide sterilization, E-Beam technology does not require nor produce large quantities of toxic/carcinogenic chemicals.
Complete this form, or email hello@nextbeam.com, and a sterilization expert will get back to you within one business day.

All of our processes meet the highest-grade industry benchmarks for quality, designed from the ground up to adhere to ISO 13485, ISO 11137, and ISO 9001 standards for medical device manufacturing, radiation sterilization, and quality. We work across industries, from medical devices to laboratory science, pharmaceuticals, and others. Our collaborative approach is designed to deliver the right solutions for your sector. We process volumes ranging from a fraction of a pallet to multiple full truckloads of product.
Stay informed with news, articles, and industry insights on sterilization.

Ready to optimize your product’s sterilization? Contact us today for a complimentary evaluation. Our sterilization expertise ensures consistent processing tailored
At NextBeam, we are deeply experienced with dose mapping and assessing products for compatibility with E-beam. Get an Expert Consultation What

At NextBeam, we are deeply experienced with medical devices and transferring them from other sterilization technologies. Contact Us to Determine

At NextBeam, we are experienced with transferring medical devices from ethylene oxide to electron beam sterilization. Contact Us to Determine If
At NextBeam, can help with transferring medical devices from gamma to electron beam sterilization. Contact Us to Determine If Your Product